Abstract
Percutaneous mitral valve therapies have emerged as an alternative option for high-risk patients with mitral regurgitation. Multiple technologies and diversified approaches are today under clinical study or in development and they can be categorised based on the anatomically and pathophysiologically addressed target. This review focuses on the different transcatheter annuloplasty techniques and explores their future perspectives.
Introduction
Mitral regurgitation (MR) is the most prevalent valve disease in the western world and it is associated with reduced life expectancy and increased risk of heart failure1,2. Surgical intervention is indicated in the presence of severe MR and symptoms of heart failure, or when patients present with reduced LV function, pulmonary hypertension, or atrial fibrillation. Mitral valve repair is the preferred method when anatomically suitable3,4. This is particularly true for primary degenerative MR (DMR).
Surgery for DMR is very safe and effective and, in relatively young patients, in the absence of comorbidities, hospital mortality is below 1%5. When performed in a timely fashion, mitral repair is able to restore life expectancy and a quality of life similar to those of the age-matched healthy population6. Surgery for secondary functional MR (FMR) carries higher risk compared to DMR: there is a 25-30% risk of MR recurrence at midterm7. Its prognostic value as well as the best surgical treatment are still debated8,9.
Overall, up to 50% of symptomatic patients with severe MR are not referred to surgery due to high surgical risk10. Percutaneous mitral valve therapies have emerged as an alternative option. Multiple technologies and diversified approaches are today under clinical study or in development. They can be categorised based on the anatomically and pathophysiologically addressed target: leaflet and chordal repair procedures, indirect and direct annuloplasty and LV remodelling devices (Figure 1).
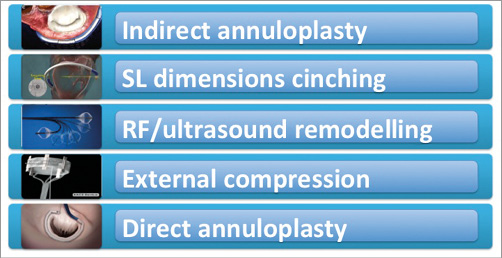
Figure 1. Classification of the different annuloplasty devices.
This review focuses on the different transcatheter annuloplasty techniques and explores their future perspectives.
Rationale and role of mitral annuloplasty in surgical mitral repair
The role of annuloplasty is to restore a normal ratio between the leaflet surface area and the annular area. Placement of an annuloplasty device during MV repair reduces mitral annular area, improves the leaflet coaptation, and prevents progressive annular dilatation and recurrent mitral regurgitation. Moreover, the stress forces acting on the valve leaflet are decreased after annuloplasty11,12, protecting from tissue tear and dehiscence of sutures.
Several surgical annuloplasty methods have been reported, including suture techniques and implantation of a prosthetic ring. Suture annuloplasty is very rarely performed today. Different annuloplasty ring devices are commonly used in surgical practice (complete or incomplete, rigid, semi-rigid or flexible), and there is no consensus as to the best technique for surgical annuloplasty: surgeon preference and experience are the main determinants in the choice of the device.
In the context of DMR, when valve leaflets are intrinsically diseased, annuloplasty is usually performed in association with leaflet or chordal repair techniques to reinforce the leaflet repair and prevent further annular dilatation. It is so far rarely performed as an isolated procedure.
In FMR, isolated annuloplasty is a very simple and effective technique and it is associated with satisfactory results, when proper patient selection is carried out13-18. The size of the ring is usually undersized by two sizes. The rationale of undersized annuloplasty in FMR is to force leaflet coaptation, by reducing the septolateral diameter. However, durability is a major concern of undersized annuloplasty in FMR, with an overall recurrence of significant MR ranging from 10% to 30% at one year, largely depending on preoperative patient selection13,14,19-23. In patients with advanced valve tethering and extreme LV remodelling, mitral valve replacement has been considered as a better alternative to undersized annuloplasty. A recent randomised trial comparing undersized annuloplasty and valve replacement in ischaemic FMR did not show any survival benefit at one year of replacement over repair (repair tended on the contrary to be associated with lower mortality). In addition, although repair was associated with an increased rate of MR recurrence, this did not translate into a clinical effect: survival, quality of life and functional status were comparable24.
In general, whether FMR should be treated with repair or replacement remains an open issue. In patients at an earlier stage of the disease and with a short clinical history of heart failure, valve repair would probably be preferable, when durable repair may be predicted by mitral anatomy.
Need for transcatheter annuloplasty techniques and description of the technologies
Currently, the unavailability of a reliable annuloplasty device is reducing the chance of eligibility for transcatheter interventions. MitraClip (Abbott Vascular, Santa Clara, CA, USA) is today the most advanced technology available for clinical use, with a proven safety and efficacy profile in selected patients with both FMR and DMR25-32. Most of the patients treated with MitraClip therapy are high-risk or inoperable patients. Improvements of symptoms and quality of life as well as MR reduction have been observed in the majority of the cases. However, about 20% of patients have residual MR and only a minority of patients undergoing MitraClip treatment have less than 2+ MR after the procedure25,29,33,34. Surgical experience has shown that long-term results of edge-to-edge in the absence of annuloplasty are suboptimal35; therefore, the absence of mitral annuloplasty is a concern regarding the durability of MitraClip treatment. Moreover, up to one third of patients screened for MitraClip are refused due to anatomical ineligibility, including annular dilatation36, suggesting that there is a clinical need for a different transcatheter mitral repair approach.
Transcatheter annuloplasty may both improve outcomes and expand therapeutic options. From a theoretical point of view, when annuloplasty devices become available and an operator will be able to combine different leaflets and annular transcatheter repair, the percutaneous technique may potentially become a true alternative to surgery in standard risk patients also.
Different catheter-based devices have made use of the coronary sinus (CS) to achieve indirect annuloplasty, whereas other devices achieve direct annuloplasty.
INDIRECT ANNULOPLASTY
The indirect annuloplasty approach is based on the anatomical proximity of the CS to the posterior mitral annulus. The CS encircles about two thirds of the mitral annulus and can be used as a route to produce tension which is transmitted to the mitral annulus, pushing the posterior annulus towards the anterior, reducing the septolateral diameter. This approach is particularly attractive because the cannulation of the CS is an easy and reproducible venous access technique. Early attempts to remodel the mitral annulus have been based on indirect annuloplasty37-39. Initial results have not been satisfactory, mainly due to suboptimal efficacy and the risk of delayed complications (including coronary occlusion).
The CARILLON® Mitral Contour System (Cardiac Dimensions, Inc., Kirkland, WA, USA) is the only technology still using this approach. It has recently obtained CE mark. The TITAN trial evaluated the clinical impact of CARILLON Mitral in HF patients with at least moderate FMR; patients in whom the device was placed and then acutely recaptured for clinical reasons (difficulty cannulating the coronary sinus, ineffective reduction in MR, or compression of a coronary artery) served as a comparator group. In contrast to the comparison group (17 patients), the implanted cohort (36 patients) demonstrated significant reductions in FMR and a corresponding reduction in LV volumes. Functional status (including walking test performance) improved markedly in the implanted patients. Coronary sinus annuloplasty was associated with delayed reverse LV remodelling and clinical improvements up to 24 months, even in acute “non-responders”40.
Although clinical benefits have been observed, this approach has a restricted applicability in the real world, mainly due to its limited efficacy (due to the fact that CS and the mitral annulus are not coplanar) and to the risk of coronary artery compression and device dislocation. Moreover, the absence of a solid surgical background is a major concern regarding long-term outcomes of the CS approach.
DIRECT ANNULOPLASTY
Direct mitral valve annuloplasty is so far the most promising approach for transcatheter mitral valve annuloplasty, since it closely reproduces the conventional surgical approach. Different technologies are under clinical investigation.
The Mitralign System (Mitralign, Inc., Tewksbury, MA, USA) was designed to perform selective plications in the mitral annulus (in P1 and P3 annular segments), by deploying pairs of transannular pledgets, which are delivered by a transfemoral retrograde approach through the mitral annulus, working from the ventricular side (Figure 2)41. The procedure is performed under live echo and fluoroscopic guidance. Preliminary clinical experience with the second-generation device has been reported in 15 high-risk patients with FMR. No periprocedural deaths were observed; in two cases mitral surgery was required. At one month, 80% of the patients had MR ≤2+. Significant quality of life improvements were observed at six months42. Patient enrolment in the multicentre CE mark trial is now complete (61 patients), but the device is still not available for clinical use. CE mark approval is expected in early 2015.
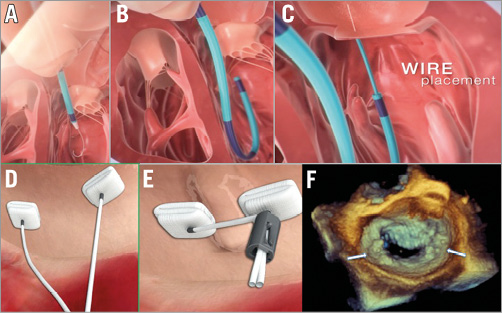
Figure 2. Direct annuloplasty with the Mitralign device: concept and procedural steps. Through the retrograde approach, the wires are placed through the mitral annulus from the ventricle to the atrium (A-C). Pairs of pledgets are then delivered in the target position (D). Plication of the annulus and positioning of the locking mechanism complete the procedure (E). Panel F shows the final result at 3D echo; pledgets are marked by two white arrows.
The Accucinch® System (Guided Delivery Systems, Inc., Santa Clara, CA, USA) is another direct annuloplasty device that also uses the retrograde transventricular approach. A series of anchors is implanted beneath the MV in the base of the LV, in the subannular space. This space is in direct contiguity with the annulus and it is relatively free from chordae. The anchors are connected by a nitinol wire. Tethering the cord under echo guidance cinches the basal LV and mitral annulus. The Accucinch System also causes remodelling of the basal portion of the LV, promoting papillary muscle approximation, and is unique in this respect. Limited clinical data are available. The feasibility and the safety of the device have been shown in 18 patients: among them, five were converted to surgery, and no 30-day deaths occurred. In the four most recent patients of this small series, an about 40% reduction of MR (quantified as regurgitant volume and effective regurgitant area) and a clinical improvement were observed43.
The Valtech Cardioband Annuloplasty System (Valtech Cardio, Inc., Or Yehuda, Israel) is the transcatheter device closest to a surgical ring (Figure 3)44. It is delivered from a transseptal approach and the implant is performed on the atrial side of the mitral annulus. The interaction with the cardiac function and the haemodynamic impact are minimal. An incomplete adjustable surgical-like sutureless Dacron band is implanted from trigone to trigone, under live echo and fluoroscopic guidance, using multiple anchor elements. The anchors are delivered starting from the anterolateral commissure progressing to the posteromedial one. After the implantation, Cardioband length may be shortened under echo guidance to improve leaflet coaptation and reduce MR (Figure 4). The CE mark trial is currently enrolling high-risk patients with FMR. Early clinical experience is promising, confirming that the Cardioband may be successfully and safely implanted in most patients. Preliminary data achieved in 24 symptomatic patients with FMR have been recently reported45, showing that Cardioband implant is associated with consistent septolateral annular dimension reduction (20% on average) and increased leaflet coaptation surface. Acute significant MR reduction was achieved in 92% of the patients (85% of the patients had MR ≤1+ at discharge); six months after the implantation 88% of the patients were in NYHA functional Class I-II and 88% of the patients had MR ≤2+. Improvements in quality of life were accordingly reported.
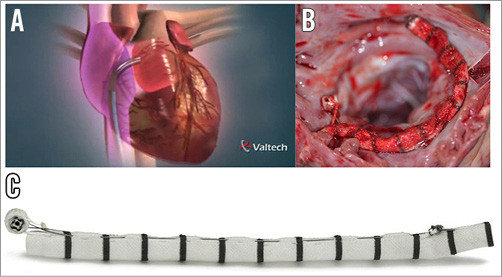
Figure 3. The Cardioband Annuloplasty System. The Cardioband device is delivered from a transseptal approach (A). B) Surgical-like aspect of the Cardioband after acute animal implant. C) Features of the device.
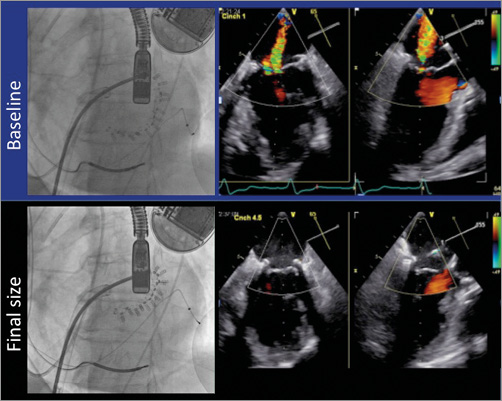
Figure 4. Fluoroscopic and echo imaging after the implantation of Cardioband at baseline and at final size, showing the abolition of mitral regurgitation and the improvement of leaflet coaptation after cinching.
Cardiac Implant’s Mitral Restriction Ring (Cardiac Implant Solutions LLC, Jacksonville, FL, USA) is another direct annuloplasty device delivered from the transseptal route, which is currently under preclinical investigation. It allows the implantation of a complete adjustable mitral ring with an internal cinching wire on the atrial annular side by means of multiple anchor elements (Figure 5). The implantable actuator is designed to enable non-invasive chronic progressive cinching also at follow-up, following the completion of tissue healing. In case of MR recurrence, the complete mitral ring may serve as a retention mechanism for a transcatheter valve-in-ring implantation. Feasibility has been reported in animal models46.
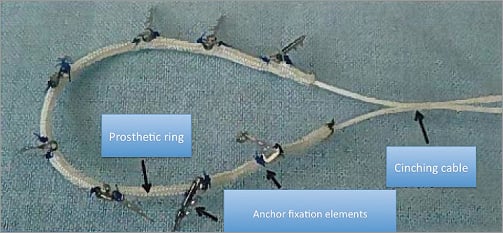
Figure 5. Cardiac Implant’s Mitral Restriction Ring device, a multi-element circumferential ring with internal cinching wire. Adapted from Kuck KH. Percutaneous Implantation of Complete Circumferential Annuloplasty Ring. EuroPCR 2014, Paris, France.
OTHER ANNULAR REPAIR TECHNIQUES
Beyond indirect CS annuloplasty and direct annuloplasty, other methods have been attempted to remodel the mitral annulus, including external compression of the atrioventricular groove47, implant of cinching devices48 and application of RF or US energy sources to shrink the annular collagen49,50. Among them, preclinical data have been reported with the ReCor (ReCor Medical, Ronkonkoma, NY, USA) device. It is a balloon catheter with a cylindrical piezoelectric ceramic transducer, which is introduced through the transseptal approach. The transducer converts electrical energy to acoustic energy, which is then delivered radially through a balloon inflated at the mitral valve annulus site, without contact. In a series of 33 dogs, one death due to energy-induced ventricular fibrillation occurred and modest reduction of annular diameters was observed50.
Reproducibility, efficacy and safety of these appealing technologies still need to be proved because they are based on completely novel concepts, without a validated and reproducible surgical or preclinical background.
The future clinical role of transcatheter annuloplasty
As multiple technologies and different approaches become available in the field of mitral valve interventions (including repair and replacement) patient selection with a proper patient-tailored approach will be crucial. When mitral replacement technologies become clinically available, it will be even more difficult to identify the ideal therapy for the individual patient. Mitral replacement may offer some theoretical advantages, such as reproducibility and applicability to the majority of patients, with a highly predictable and less technically demanding procedure. However, transcatheter mitral valve repair, including annuloplasty, although more complex and technically challenging, is more physiological and is associated with a superior safety profile, as compared to replacement, since it does not involve a heterologous tissue implant and does not require anticoagulation. The overall balance between advantages and disadvantages of the two approaches introduces the need for a patient-specific tailored approach.
In DMR patients, annuloplasty has to be considered as an adjunctive therapy in combination with leaflet repair. Currently, MitraClip is the only transcatheter device available for clinical use in the treatment of DMR: outcomes could certainly benefit from the association of an annuloplasty (similarly to the surgical approach), to achieve better acute results and improve repair durability.
In FMR patients, annuloplasty might represent a stand-alone procedure. Therefore, it will be extremely important to identify the ideal candidate who could benefit most from each device or technique: MitraClip, CS annuloplasty, direct annuloplasty or transcatheter mitral valve implantation. Patients with predominant annular dilatation and limited valve tethering could be the ideal candidates for annuloplasty, while in the presence of predominant leaflet tethering MitraClip could still represent the more appropriate therapy. A combination of different technologies may represent a better option in selected FMR patients: surgical literature reported improved repair durability with the association of edge-to-edge to annuloplasty in patients with FMR and advanced tethering17. Some open issues block the evolution of combination therapy, including the cost of the two procedures, the potential risk of mitral stenosis and the definition of the ideal sequence of the two-step approach.
The clinical introduction of transcatheter mitral technologies could promote the adoption of an “early indication” strategy. When patients are treated in a too advanced clinical status, outcomes become poor and transcatheter mitral procedures are unable to modify the clinical course of the disease and to influence the prognosis. The impact of the intervention will be much more efficient when executed early in the clinical course of the disease. Only a very safe procedure can justify a transcatheter “early indication” approach. In fact, when considering an early indication, beyond efficacy, safety plays a dominant role. Transcatheter direct annuloplasty may represent the ideal procedure that could be used as a safe first-line therapy in FMR patients. Considering an early indication approach, if comparable efficacy is proven, direct annuloplasty may present some advantages as compared to MitraClip: the possibility of valve-in-ring implantation and not precluding the option of surgery.
Conclusions
In conclusion, direct mitral annuloplasty is nowadays the most promising technology to improve the results of mitral valve interventions. These devices have the potential to expand the overall number of patients who could benefit from a transcatheter mitral intervention and to improve the efficacy and durability of the currently available techniques, by the combination of annular and leaflet repair in the same patient.
When reliable annuloplasty and valve replacement devices become clinically available, transcatheter mitral interventions may become a real alternative to surgery, even in intermediate-risk patients, especially in the context of FMR.
Conflict of interest statement
F. Maisano is a consultant for Abbott Vascular and for Valtech Cardio. The other authors have no conflicts of interest to declare.

