Abstract
The treatment of mitral regurgitation has changed in recent years due to the advent of interventional techniques, mostly the percutaneous edge-to-edge repair, and, more recently, annuloplasty and chordal replacement. In the future, more advances are expected from further development of interventional techniques, careful evaluation and better patient selection.
Introduction
Mitral regurgitation (MR) is a frequent valvular disease and its prevalence is increasing due to the ageing of the population. During the last 10 years, the development of transcatheter techniques has increased the “therapeutic offer” beyond cardiac surgery and medical therapy. Here, we shall discuss the techniques of transcatheter mitral valve repair (TMVR), concentrating on those for which clinical experience is already available and which already have CE mark approval.
General comments on mitral regurgitation
The mitral valve is a complex entity where several components interact – valve leaflets, subvalvular apparatus, annulus, and left ventricle (LV). There are two main types of MR, primary and secondary, which have different pathophysiology and anatomic aspects and, as a consequence, require different treatments1,2. Observational studies have shown that many patients are denied surgery by their cardiologist before any consideration of intervention because the risk of surgery is considered prohibitive, ignoring the poor outcome of the natural history3.
General principles for transcatheter mitral interventions
The complexity and heterogeneity of mitral valve disease explains why the development of TMVR took longer than that of transcatheter aortic valve implantation (TAVI). Evaluation should be carried out by a multidisciplinary Heart Team4,5. The decision making for transcatheter techniques will be as an alternative to surgery in high-risk patients and to heart transplant, LV assist device or medical therapy in inoperable patients.
Specific transcatheter interventions
Because of the heterogeneity of lesions and mechanisms, several TMVR therapies have been developed which attempt to replicate the most effective surgical techniques – leaflet repair, annuloplasty, chordal replacement. As for surgery, most of these therapies are complementary to each other and may be used as combined therapy. Multiple devices have been invented, but only a few have progressed to the CE mark stage.
LEAFLET REPAIR
MITRACLIP® THERAPY
MitraClip (Abbott Vascular, Santa Clara, CA, USA) therapy, the most popular technique, was inspired by the Alfieri stitch surgical technique (Figure 1A). The clip grasps the free edge of both leaflets forming a double orifice valve. A recent improvement is the MitraClip® NT (Abbott Vascular) which allows improved grasping, deeper leaflet insertion, and more stable fixation.
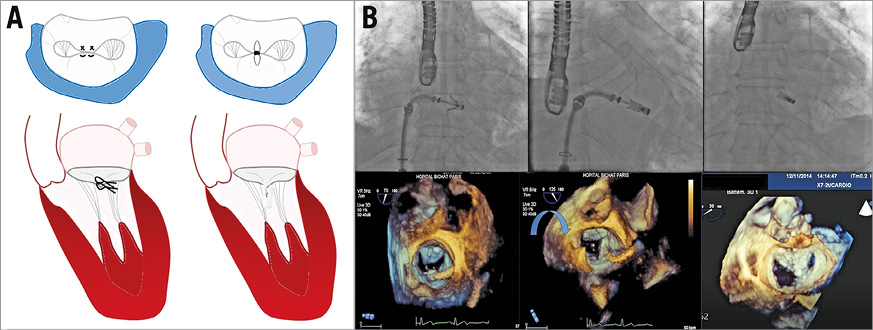
Figure 1. MitraClip therapy. A) Alfieri stitch surgical technique compared to MitraClip. B) Fluoroscopic and 3D echocardiographic images showing different steps of the procedure: positioning of the clip, closing of the clip and final result.
The technique requires a transseptal approach performed at a septal puncture site individualised according to the nature of MR. Navigation in the left atrium (LA) uses straddling and steering capacities in order to allow the positioning and alignment of the device perpendicularly to the commissural line, and finally to grasp the leaflets at the desired site (Figure 1B, Moving image 1-Moving image 3). If needed, the clip can be repositioned and/or additional clips can be implanted, the goal being a sufficient and stable capture of the leaflet, and a significant reduction in the degree of MR without creating mitral stenosis. The procedure is usually well tolerated. The technique is more challenging in primary MR, although improved results are expected with the use of the MitraClip NT which allows the capture of a greater volume of tissue. There is a learning curve shown by a higher success rate (>90%) in the current series, with less residual MR (MR ≤2+ in around 90% of cases)6.
To date, over 48,000 patients have been treated worldwide. Primary MR represents only 30% of MitraClip implantations performed worldwide, while it is the almost exclusive indication in the USA where this is the only indication accepted by the FDA.
The randomised EVEREST (Endovascular Valve Edge-to-Edge Repair Study) II trial, which was performed in the early phase of the experience with the device, compared MitraClip implantation to surgery in 279 patients who were surgical candidates, 2/3 having primary MR and 1/3 secondary MR. MitraClip implantation showed better safety than surgery, but more residual MR (MR >2+ in 57% vs. 24%) after five years. Survival was not different between the two groups but the need for surgery was higher after MitraClip implantation (27.9% vs. 8.9%) related to a higher incidence of MR 3+/4+ (12.3% vs. 1.8%). The actuarial curves separate early due to procedural failures and then remain parallel showing the durability of the results over time. Functional results are equivalent. LV remodelling occurred in both arms and was slightly better in the surgical arm7.
A number of registries, mostly in high-risk patients with secondary MR8-12, but also more recently with primary MR12, confirm the safety of the procedure, and show improvement in symptoms and quality of life; however, the majority of patients still have mild to moderate MR. These registries consistently suggest functional improvement after MitraClip implantation but survival benefit remains to be proven13. Surgery is feasible after failure of the clip but it is uncertain if the attempted MitraClip implantation affects the performance and quality of surgical repair, the success rate of which varies from 20 to 50% 14.
Large registries9,10 have allowed identification of the predictors of one-year outcomes which are multiple: age >75 years and advanced stage of the disease (NYHA Class IV, severe pulmonary hypertension, LV ejection fraction [LVEF] <30%). Some reports have suggested that MitraClip implantation may allow symptomatic improvement in patients with very low LVEF but follow-up is lacking14. Other markers of poor prognosis such as high BNP, and the presence of severe tricuspid regurgitation, especially if accompanied by severe RV dysfunction or severely enlarged LV and RV dysfunction, may also help to identify patients for whom it is too late for valve intervention and for whom transplantation may be the best option, after discussion by the Heart Team.
Regarding procedural failure, the outcome is related to the magnitude of the residual MR and every attempt should be made during the procedure to minimise it. Nonetheless, it is necessary to assess individually the balance between less residual MR and greater residual gradients/smaller mitral valve areas when multiple clipping is considered.
The use of the MitraClip has been reported in small series of inoperable patients with acute MR due to papillary rupture or cardiogenic shock. Case reports also suggest the efficacy of the technique after failure of surgical annuloplasty15, or to reduce systolic anterior motion (SAM) in hypertrophic cardiomyopathy.
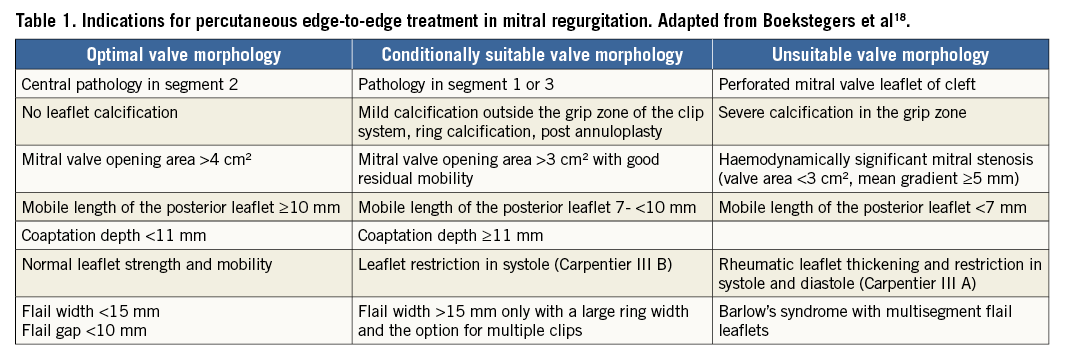
The current guidelines consistently state that MitraClip implantation may be considered in inoperable patients with primary MR if life expectancy is acceptable. However, there is a discrepancy between guidelines on the indications in secondary MR: the 2016 ESC/EACTS Guidelines on management of heart failure16 state that MitraClip implantation may be considered in patients with secondary MR refractory to medical therapy, with the main objective of reducing symptoms, but they state that more evaluation is needed. Conversely, the 2017 ACC/AHA Valve Guidelines state that MitraClip implantation may be considered only in patients with primary MR4. In addition, the anatomic criteria defined in the EVEREST trial have been revisited in a consensus paper taking into account that in contemporary practice the majority of patients are treated beyond the anatomic criteria described in the EVEREST trial17,18 (Table 1).
However, suboptimal results have been reported when edge-to-edge surgical repair is used alone, and thus currently it is used in combination with surgical annuloplasty in most cases. Likewise, a combination of percutaneous therapies (i.e., MitraClip and annuloplasty) might be advisable in these patients.
ANNULOPLASTY
Annuloplasty percutaneous therapies are intended to reproduce the surgical annuloplasty which is intended to correct annular dilatation supporting other valve repair techniques and to prevent further annular dilatation.
INDIRECT ANNULOPLASTY
Carillon® Mitral Contour System®
The Carillon® Mitral Contour System® (Cardiac Dimensions Inc., Kirkland, WA, USA) is intended to modify the posterior mitral annulus geometry, reducing the dimensions of the mitral annulus and the severity of functional mitral regurgitation (Figure 2A). The Carillon is a self-expanding device which consists of two anchors connected by a nitinol wire. Overall, it is a relatively easy procedure which is guided by fluoroscopy. The device is implanted via the jugular vein in the coronary sinus and great cardiac vein, adjacent to the posterior and lateral part of the mitral annulus. Once placed, tension is exerted on the system leading to a cinching of the posterior annulus (Figure 2B, Moving image 4). In case of suboptimal results or complications, the device can be replaced or removed19.
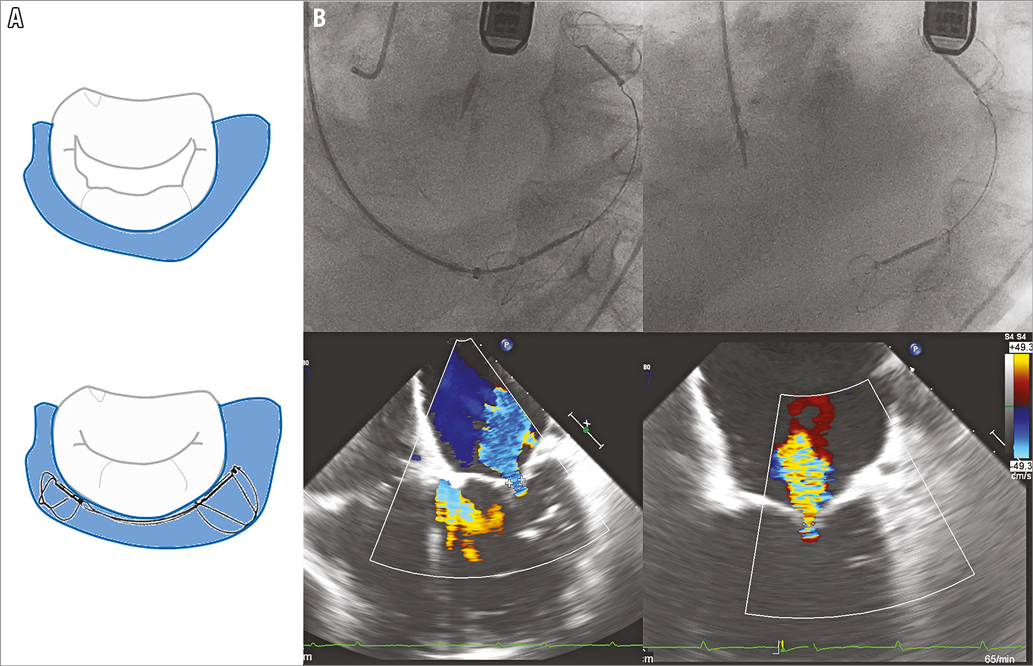
Figure 2. The Carillon device. A) Cinching of the posterior annulus with the Carillon device. B) Fluoroscopic and echocardiographic images showing the deployment and final result of the Carillon therapy (courtesy of M. Haude).
More than 600 devices have been implanted to date. Three main, non-randomised trials have evaluated the results of the Carillon device, which received the CE mark in 2011 - the AMADEUS19, the TITAN20 and the TITAN II trials21, including a total of 137 patients with dilated ischaemic or non-ischaemic cardiomyopathy and functional MR. A successful implantation of the device resulted in a modest but significant reduction of the septal-lateral diameter of the mitral annulus (by 13%) and, consequently, a 50% decrease in the regurgitant volume, an improvement of at least one grade of MR in up to 80% of patients at 12 months, and a discrete favourable LV remodelling. This translated into an improvement of the NYHA class (from III to II) in most patients and an increase in the distance walked during the 6-minute walking test.
However, the rate of procedural success was relatively low (60 to 83%), and initially the risk of major adverse events was about 13%. Although the rate of procedural complications was dramatically reduced in the TITAN and TITAN II trials in which enhanced generations of the device were used, concerns remain. The first is the relatively low success rate, which might be mostly due to anatomical variations. Indeed, the coronary sinus runs, in its proximal or distal part, superiorly or inferiorly to the mitral annulus in the majority of patients and might be at some distance from the sinus in the axial plane22. This distance might be more important in patients with mitral regurgitation. Second, in roughly 70% of patients the circumflex artery courses between the coronary sinus and the mitral annulus, increasing the risk of impingement of the circumflex artery.
DIRECT ANNULOPLASTY
Cardioband
The Cardioband (Valtech Cardio, Or Yehuda, Israel) replicates surgical band annuloplasty (Figure 3A). The transseptal route is used to introduce a steerable guide and a catheter-delivered nitinol band which is adjustable.
It is likely that the optimal anatomic criteria for echographic selection will be quite similar to those for surgical ring annuloplasty. In addition, the screening necessitates a careful CT examination to locate the most appropriate transseptal puncture site, determine device size and the number of anchors to be implanted, identify the working views for implantation, show the relationship between mitral annulus and the circumflex coronary artery, and eliminate annular calcifications.
After the transseptal puncture, the Dacron band is progressively deployed from commissure to commissure over the posterior part of the mitral annulus. Multiple anchors are screwed into the mitral annulus with fluoroscopic and transoesophageal echocardiography (TOE) guidance using X-Plane and 3D en face views. When complete anchoring is achieved, cinching is progressively applied under echo guidance to reduce MR (Figure 3B, Moving image 5-Moving image 7).
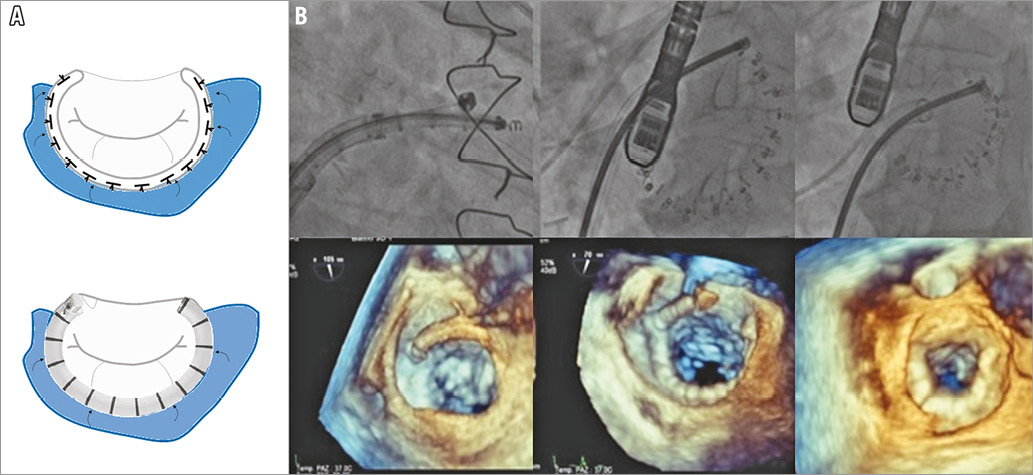
Figure 3. The Cardioband. A) The Cardioband replicates surgical band annuloplasty. B) Fluoroscopic and 3D echocardiographic images showing anchor implantation and final cinching of the mitral annulus.
As is the case with MitraClip, the technique is well tolerated. Its performance is more demanding than that of the MitraClip and there is a steep learning curve.
The total experience in around 150 high-risk patients with secondary MR suggests that the technique is feasible (success rate >90%) and safe, since no significant device-related complications have been reported23,24. It provides significant and consistent reduction in MR: 90% of patients have MR ≤2+ which is related to a significant reduction in annular size (septolateral dimension: –30%)25. MR reduction and clinical improvement are stable at 12 months: 92% of patients had MR ≤2+ and 77% were in NYHA Class I or II with a parallel improvement in objective testing such as the 6MWT24.
One of the potential advantages of this technique is that it leaves the options open for future interventions.
Mitralign
The Mitralign Percutaneous Annuloplasty System (Mitralign Inc, Tewksbury, MA, USA) is another direct annuloplasty system aimed at reproducing the surgical repair of Paneth (Figure 4A). The procedure is performed under fluoroscopic and TOE guidance via a retrograde arterial approach of the mitral annulus from the LV. Puncture of the mitral annulus is performed using radiofrequency wires and pledgets are inserted (one or two pairs at the level of each commissure) and then assembled to cinch the annulus. Finally, they are locked using a stainless steel lock (Figure 4B).
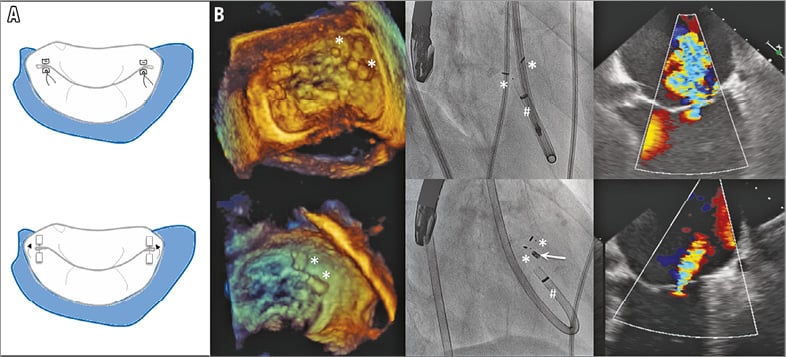
Figure 4. The Mitralign device. A) The Mitralign device compared to the Paneth mitral repair technique. B) Fluoroscopic and echocardiographic images showing device deployment and final results. # denotes the guide catheter and white arrow the stainless steel lock.
A total of 71 high-risk patients with secondary MR have been reported26. The device success rate was 70% due to an initial learning curve and iteration of the devices. No death was device-related but tamponade occurred in 8%. At six months, 77% of patients had functional improvement, left ventricular remodelling was initiated but MR reduction, by 1.3 grades on average, only occurred in 50% of patients. This is related to a modest (12%) reduction in septal-lateral annular diameter.
CHORDAL REPLACEMENT
NeoChord DS1000
The NeoChord DS1000 (NeoChord Inc, St. Louis Park, MN, USA) replicates a proven effective surgical therapy. It consists in the off-pump insertion of artificial heart chords using the NeoChord DS 1000 System, through a transapical approach which restores the coaptation of leaflets on a beating heart (Figure 5A, Figure 5B). Under echocardiographic guidance, the leaflets are grasped using the expandable jaws of the device and a suture is attached to the leaflet and pulled through the cardiac apex (Moving image 8-Moving image 10). Once the length of the chord has been selected, the delivery system is removed and the second suture is secured to the apex27.
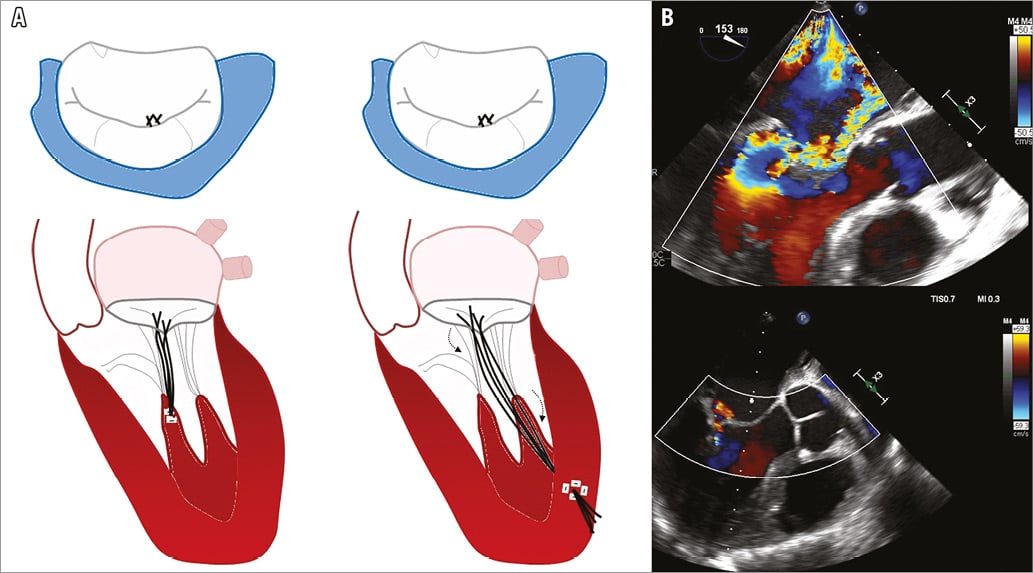
Figure 5. The NeoChord DS1000. A) NeoChord compared to surgical artificial chordae implantation. B) Doppler echocardiographic images showing the final result of the procedure (courtesy of J.F. Obadia).
The proof of concept of this therapy was demonstrated in the TACT (Transapical Artificial Chordae Tendinae) trial27 including 30 patients with severe MR attributable to isolated posterior MV prolapse without annulus dilation. Acute procedural access, which was defined as the placement of at least one NeoChord and a reduction of MR to ≤2+, was achieved in 86% of patients. Major complications occurred in 26% of patients, with a 20% reoperation rate (all of them underwent surgical mitral valve repair) and 3.3% death rate. Of note, at 30 days, only 56% of patients with successful procedure maintained a <2+ grade MR.
Several lessons were learned during this study: the success rate of the procedure may be increased using a posterolateral transapical access site (rather than the LV apex), multiple NeoChords are necessary and an accurate selection process is mandatory to achieve success. In 2012, this therapy received CE mark approval and more than 500 patients have been treated to date.
Although the technique allows acute evaluation in more physiological conditions while preserving the possibility of surgical repair, several challenges remain to be addressed: the use of a transapical approach, which increases the risk of complications; to determine the exact positioning and length of neo-chordae might be difficult; the impact of the apical fixation of the chordae compared to the surgical papillary muscle fixation and the durability are unknown; and the potential risk of recurrence without the combination of other techniques (such as annuloplasty which is usually performed in the surgical setting) might be higher.
Future considerations
As will be shown in a subsequent article in the journal, multiple devices are either at experimental, FIM, or early clinical development stages enriching the armamentarium of TMVR.
– Imaging will be a key component in the development of TMVR. The collaborative work between interventionists and imagers should be reinforced and training in imaging will be key for interventionists. Multimodality imaging combining echocardiography and CT will allow better patient selection and optimal procedural guidance to improve the efficacy and ease of TMVR. The search for simplification of the procedure should continue, researching alternatives to general anaesthesia which is made necessary by the use of TOE.
– New indications should be based on evidence coming from RCTs and real-life registries using well-defined endpoints28. The first step will be to establish the role of any valvular intervention in patients with secondary MR. Several RCTs are ongoing comparing optimised medical management with MitraClip implantation: the MITRAFR trial has now been completed and COAPT (NCT01626079) and RESHAPE-HF 2 (NCT02444338) are in the final phase of enrolment. Finally, other randomised trials with similar designs will be completed in the coming years– REDUCE FMR (NCT02325830) and the CARILLON trials (NCT03142152) with the Carillon device, and the REPAIR trial (NCT02703311) with Cardioband.
– The effectiveness of the annuloplasty techniques should be assessed in larger populations with follow-up. The effectiveness of chordal replacement also deserves further studies. Two ongoing randomised trials comparing the transcatheter insertion of neo-chordae to surgical mitral valve repair (the ReChord trial, NCT02803957, and the MITRACHORD, NCT02829749) will shed more light on the results of this therapy.
– The efficacy, short- and long-term, of the MitraClip should be evaluated in lower-risk (high and intermediate) patients with primary MR in comparison with the surgical gold standard.
– Surgical mitral valve repair uses a combination of techniques adapted to lesions and type of dysfunction and, in the future, it should be possible to replicate this with TMVR. As an illustration, combining annuloplasty with MitraClip may increase the indications and improve efficacy in secondary MR. Initial clinical experience has also shown that one device can be used in case of failure of the other. In primary MR, annuloplasty could also be combined with MitraClip or chordal replacement. Experience will tell us how and when these techniques should be combined, keeping in mind the inherent consequences in terms of complexity and cost. Mitral repair techniques can also be performed to treat MR in patients with severe primary MR undergoing TAVI or, more often, in combination with percutaneous treatment of tricuspid regurgitation. Here again the clinical experience is very preliminary29.
– In the surgical treatment of MR, at least of primary MR, repair is preferred over replacement when a durable result can be expected. It is difficult today to compare the results of TMVR, which has been performed in almost 50,000 patients with over five years of follow-up, with those of transcatheter valve replacement where the total experience is probably around 200 with follow-up being in months. At first glance, TMVR has several advantages over valve replacement: it restores more natural haemodynamics and safety is good. However, TMVR is technically more complex and works only in selected patients. Finally, MR reduction is less predictable than after replacement. It is likely that the two techniques will be complementary as is the case in the surgical field.
Conclusions
The transcatheter mitral valve techniques were introduced approximately 10 years ago but have already revolutionised the treatment of MR as now, in Germany, more patients are treated with transcatheter than with surgical valve repair and, importantly, a higher number of patients are treated by one or other approach. In the future, changes will occur due to progress in technology, imaging and patient selection, with any change in practice being based on evidence.
Conflict of interest statement
A. Vahanian has served as a consultant to Abbott and Edwards Lifesciences (modest amount). H. Ince has received research grants from Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. TOE 4-chamber view showing a severe prolapsus of the posterior leaflet resulting in severe mitral regurgitation.
Moving image 2. TOE intercommissural and long-axis mid-oesophageal views showing the grasping of the leaflets.
Moving image 3. 3D TOE images showing the final result of the MitraClip procedure.
Moving image 4. Fluoroscopy images showing the final deployment of the Carillon device. Courtesy of M. Haude.
Moving image 5. Fluoroscopic images showing the final result of the Cardioband procedure.
Moving image 6. 2D and 3D TOE images showing the implantation of the Cardioband device.
Moving image 7. 3D TOE images showing the reduction of the mitral annulus dimensions after cinching of the Cardioband device.
Moving image 8. Insertion of an artificial chord using the NeoChord DS1000 System in a model. Courtesy of J.F. Obadia.
Moving image 9. Long-axis TOE images showing a severe posterior prolapsus resulting in severe mitral regurgitation. Courtesy of J.F. Obadia.
Moving image 10. Long-axis TOE images showing the final result of the NeoChord therapy. Courtesy of J.F. Obadia.
Supplementary data
To read the full content of this article, please download the PDF.
TOE 4-chamber view showing a severe prolapsus of the posterior leaflet resulting in severe mitral regurgitation.
Long-axis TOE images showing the final result of the NeoChord therapy. Courtesy of J.F. Obadia.
TOE intercommissural and long-axis midoesophageal views showing the grasping of the leaflets.
3D TOE images showing the final result of the MitraClip procedure.
Fluoroscopy images showing the final deployment of the Carillon device. Courtesy of M. Haude.
Fluoroscopic images showing the final result of the Cardioband procedure.
2D and 3D TOE images showing the implantation of the Cardioband device.
3D TOE images showing the reduction of the mitral annulus dimensions after cinching of the Cardioband device.
Insertion of an artificial chord using the NeoChord DS1000 System in a model. Courtesy of J.F. Obadia.
Long-axis TOE images showing a severe posterior prolapsus resulting in severe mitral regurgitation. Courtesy of J.F. Obadia.

