Abstract
Mitral regurgitation (MR) is the most prevalent valvular heart disease and, when left untreated, results in reduced quality of life, heart failure, and increased mortality. Mitral valve transcatheter edge-to-edge repair (M-TEER) has matured considerably as a non-surgical treatment option since its commercial introduction in Europe in 2008. As a result of major device and interventional improvements, as well as the accumulation of experience by the interventional cardiologists, M-TEER has emerged as an important therapeutic strategy for patients with severe and symptomatic MR in the current European and American guidelines. Herein, we provide a comprehensive up-do-date overview of M-TEER. We define preprocedural patient evaluation and highlight key aspects for decision-making. We describe the currently available M-TEER systems and summarise the evidence for M-TEER in both primary mitral regurgitation (PMR) and secondary mitral regurgitation (SMR). In addition, we provide recommendations for device selection, intraprocedural imaging and guiding, M-TEER optimisation and management of recurrent MR. Finally, we provide information on major unsolved questions and “grey areas” in M-TEER.
Introduction
Mitral regurgitation (MR) is a highly prevalent disease with approximately 10% of individuals aged 75 years or more having clinically relevant MR1. When left untreated, the consequences of severe MR include left ventricular dysfunction, reduced cardiac output and pulmonary congestion, leading to heart failure (HF) symptoms, reduced quality of life and increased mortality2. Many patients with severe MR are inoperable or at increased surgical risk; accordingly, transcatheter therapies were developed to address undertreatment of this patient population. Since the first human clinical experience of mitral valve transcatheter edge-to-edge repair (M-TEER) in 20033 and its subsequent CE (European Conformity) mark in 2008 and US Food and Drug Administration (FDA) approval for primary MR (PMR) in 2013 and for secondary MR (SMR) in 2019, M-TEER has become an established alternative to surgery. Over 150,000 patients around the globe have been treated with M-TEER to date.
The present manuscript summarises the most important aspects of M-TEER, which is being performed by an increasing number of hospitals worldwide4. A state-of-the-art review including preprocedural patient evaluation, device updates, and practical recommendations for patient and device selection in both PMR and SMR is provided. In addition, tips and tricks for interventionalists to optimise M-TEER results are included, and the major unsolved questions and “grey areas” are highlighted.
Preprocedural patient evaluation and decision-making
Patient care pathway for M-TEER
Patients with evidence for relevant MR require precise clinical and imaging assessment at a heart valve centre with dedicated competencies and experience. Clinical evaluation includes a physical examination with a search for HF signs and grading of the symptomatic status according to the New York Heart Association (NYHA) classification. Patients’ medical history and comorbidities, in particular with respect to previous HF hospitalisations, as well as hepatic and renal function, will influence the treatment decision. Quality of life should be assessed, preferably using standardised HF questionnaires. Objective functional assessment may include a 6-minute walk test, cardiopulmonary exercise testing and spiroergometry, along with the use of wearable monitors for continuous tracking of physical activity. Additional exams may be considered in case of discrepancies between clinical and imaging findings, as well as in apparently asymptomatic patients.
Transthoracic echocardiography (TTE) is the key technique to confirm the diagnosis of relevant MR, clarify its mechanism and severity, estimate pulmonary artery pressures, and evaluate concomitant heart valve disease as well as left ventricle (LV) and right ventricle (RV) functions. While TTE is commonly used as the primary echocardiographic imaging method, transoesophageal echocardiography (TOE) is required in almost all potential M-TEER candidates for a better and more precise mitral valve (MV) characterisation. As the regurgitation grade can be underestimated at rest, pharmacological, or better yet, physiological exercise echocardiography might be useful for the assessment of changes in mitral regurgitant volumes (RVol) and pulmonary pressures during peak exercise, but this concept needs further investigation5.
Coronary angiography is recommended to evaluate the need for concomitant coronary revascularisation. Alternatively, coronary computed tomography angiography may be used in selected patients with low likelihood of coronary artery disease and stable sinus rhythm. Right heart catheterisation should be considered particularly in patients with reduced RV function, pulmonary hypertension or severe tricuspid regurgitation (TR) for risk assessment and evaluation of the haemodynamic consequences of valvular heart disease (VHD).
After the above-mentioned diagnostic steps, a multidisciplinary Heart Team will evaluate each case individually. The Heart Team should consist, at least, of an interventional cardiologist with experience in MV therapy, a cardiac surgeon with experience in MV repair and replacement, and an imaging specialist with expertise in interventional imaging. While the Heart Team should include a heart failure specialist for SMR patients, their role for PMR patients is less defined. The Heart Team aggregates the clinical information with the diagnostic results, incorporating the values and expectations of the informed patient and his or her relatives, when appropriate. In patients with SMR, the Heart Team evaluates and optimises the existing medical and device HF therapy before making a decision on any MV therapy. A simplified decision tree, which is based on current European valvular guidelines, is shown in Figure 1. Risk stratification supports the decision-making. The Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) score (http://riskcalc.sts.org/stswebriskcalc/calculate) and the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II; http://www.euroscore.org/calc.html) help to predict early postoperative mortality6. The value of dedicated risk scores for predicting 1-year survival after M-TEER will be discussed below. In inoperable or high-risk patients with PMR, as well as in those with SMR without the need for surgical revascularisation, M-TEER should be evaluated providing anatomical feasibility (Figure 1). Palliative care should be considered in patients with limited life expectancy (<1 year), while left ventricular assist device (LVAD) implantation or heart transplantation (HTx) may represent a more appropriate option for selected patients with advanced HF and MR.
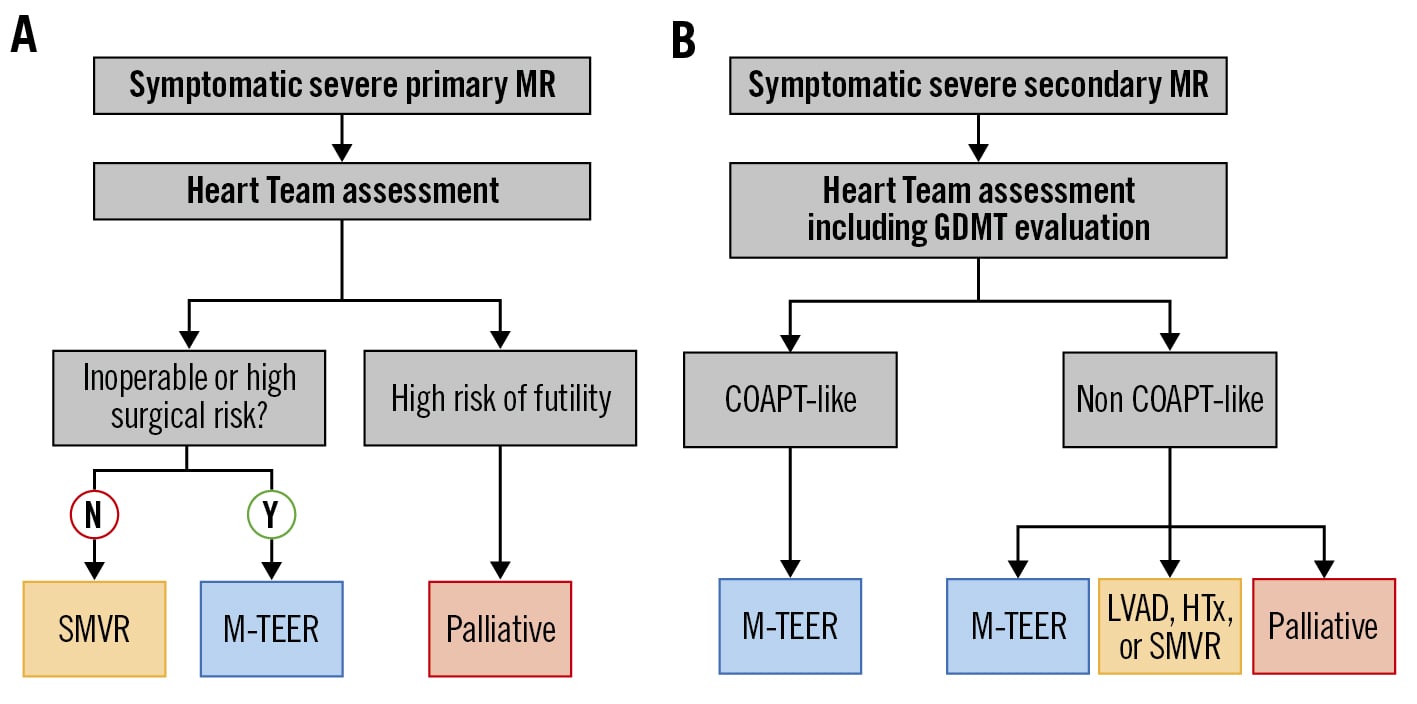
Figure 1. Patient stratification and simplified guideline recommendation. Therapeutic strategies for patients with (A) symptomatic severe primary mitral regurgitation (MR) or (B) symptomatic severe secondary MR. COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; GDMT: guideline-directed medical therapy; HTx: heart transplantation; LVAD: left ventricular assist device; M-TEER: mitral valve transcatheter edge-to-edge repair; N: no; SMVR: surgical mitral valve repair or replacement; Y: yes
MR aetiology
Correct evaluation of MR aetiology is of key importance due to its impact on prognosis and management, as well as procedural planning7. PMR, also termed degenerative or organic MR, relates to leaflet or subvalvular apparatus lesions. Leaflet degeneration (leaflet prolapse or flail leaflets; Carpentier type II) is the most prevalent aetiology in Western countries, while leaflet restriction (rheumatic, postinflammatory or post-radiotherapy; Carpentier type IIIa) is a frequent cause of PMR in low-income countries89.
SMR, also termed functional MR, is usually due to either annular dilation with normal leaflet motion (Carpentier type I) or leaflet motion restriction due to left ventricular remodelling and dilation. In the latter case, the restriction occurs in systole, defining MR Carpentier type IIIb. Of note, the 2 mechanisms can coexist in patients with SMR, in whom papillary muscle dysfunction and dyssynchrony may also play a role. SMR can be further classified according to its aetiology as ischaemic, non-ischaemic, or atrial. In ischaemic SMR, regional LV wall dysfunction results in remodelling, with subsequent restriction of leaflet motion through tethering. In more severe cases, global LV hypokinesia and left ventricular dilatation can occur. Non-ischaemic ventricular SMR is most frequently encountered in patients with severe dilated cardiomyopathy resulting in annular dilatation, papillary muscle displacement and finally MV leaflet restriction. Atrial SMR, which has recently gained increasing scientific interest, is caused by left atrial enlargement due to, e.g., persistent or chronic atrial fibrillation (AF); left ventricular function is typically preserved in these patients10.
Quantification of MR
The grading of MR relies on a multiparametric integrative approach, including qualitative, semi-quantitative and quantitative parameters (Table 1). The main quantitative echocardiographic parameters defining severe MR are a vena contracta ≥7 mm, a regurgitant fraction ≥50%, an effective regurgitant orifice area (EROA) ≥40 mm2, and an RVol ≥60 ml.
The EROA derived from the proximal isovelocity surface area (PISA) method often underestimates the true size of the regurgitant orifice in SMR due to its semi-lunar shape. Therefore, lower thresholds of EROA ≥30 mm2 and RVol ≥45 ml can be considered due to an elliptical EROA or low-flow conditions in SMR patients. However, the validity and applicability of these thresholds have been questioned: Table 2 demonstrates the problematic use of the RVol thresholds in a COAPT-like patient with ventricular SMR and reduced left ventricular ejection fraction of 35%. The mathematic modelling reveals that the required RVol thresholds of 45 or 60 ml for defining severe SMR result in very low cardiac outputs, which are barely or not at all compatible with survival. This theoretical assumption demonstrates that PISA-based EROA and RVol assessments should be applied with caution in patients with SMR, and a multiparametric approach is required for identifying suitable M-TEER candidates.
TOE planimetry of the 3D vena contracta area represents a useful complementary method that has been linked to prognosis11. A more detailed description of the MV evaluation by echocardiography is available in the latest respective European and American documents811.
After M-TEER, echocardiographic imaging should be repeated at follow-up visits to assess the durability of the procedural result. Post-procedural MR should be graded using an integrative approach, similar to the preprocedural echocardiographic baseline assessment. PISA evaluation after M-TEER has been debated, since the presence of a clip may prevent accurate, reproducible measurements of multiple, sometimes eccentric, regurgitant jets with non-hemispheric proximal flow convergence12. RVol and regurgitant fraction after M-TEER can be alternatively assessed using Doppler haemodynamic and volumetric analysis.
Table 1. Quantification of severe MR.
| Primary mitral regurgitation (PMR) | Secondary mitral regurgitation (SMR) | ||
|---|---|---|---|
| Qualitative | Mitral valve morphology | Flail leaflet, ruptured papillary muscle, severe retraction, large perforation | Normal leaflets but with severe tenting, poor leaflet coaptation |
| Colour flow jet area | Large central jet (>50% of LA) or eccentric wall impinging jet of variable size | ||
| Flow convergence | Large throughout systole | ||
| Continuous wave Doppler jet | Holosystolic/dense/triangular | ||
| Semi-quantitative | Vena contracta width (mm) | ≥7 (≥8 mm for biplane) | |
| Pulmonary vein flow | Systolic flow reversal | ||
| Mitral inflow | E-wave dominant (>1.2 m/s) | ||
| TVI mitral/TVI aortic | >1.4 | ||
| Quantitative | EROA (2D PISA, mm2) | ≥40 mm2 | ≥40 mm2 (may be ≥30 mm2 if elliptical EROA) |
| Regurgitant volume (mL/beat) | ≥60 mL | ≥60 mL (may be ≥45 mL if low-flow conditions) | |
| Regurgitant fraction (%) | ≥50% | ||
| Structural | Left ventricle | Dilated (ESD ≥40 mm) | Dilated |
| Left atrium | Dilated (diameter ≥55 mm or volume ≥60 mL/m2) | Dilated | |
| EROA: effective regurgitant orifice area; ESD: end-systolic diameter; LA: left atrium; MR: mitral regurgitation; PISA: proximal isovelocity surface area; TVI: time-velocity integral | |||
Table 2. Three echocardiographic scenarios demonstrating the problematic use of the regurgitant volume thresholds in a typical, “COAPT-like” patient with ventricular SMR.
| Typical, “COAPT-like” patient with ventricular SMR (assumptions: 35% LVEF, 200 mL LVEDV, 75 bpm heart rate, 2 m2 BSA) | ||||||
|---|---|---|---|---|---|---|
| Stroke volume (mL) |
RVol (mL) |
Regurgitant fraction (%) |
Forward stroke volume (mL) | Cardiac output(L/min) | Cardiac index(L/min/m2) | |
| Scenario 1 RVol 30 mL/EROA 0.2 cm2 |
70 | 30 | 43 | 40 | 3.0 | 1.5 |
| Scenario 2 RVol 45 mL/EROA 0.3 cm2 |
70 | 45 | 64 | 25 | 1.9 | 0.9 |
| Scenario 3 RVol 60 mL/EROA 0.4 cm2 |
70 | 60 | 86 | 10 | 0.8 | 0.4 |
| BSA: body surface area; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; EROA: effective regurgitant orifice area; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; RVol: regurgitant volume; SMR: secondary mitral regurgitation | ||||||
M-TEER systems
Two M-TEER devices are currently approved in Europe for minimally invasive treatment of the MV: the MitraClip (Abbott) and PASCAL (Edwards Lifesciences) systems. The main differences between the 2 platforms are illustrated in Figure 2.
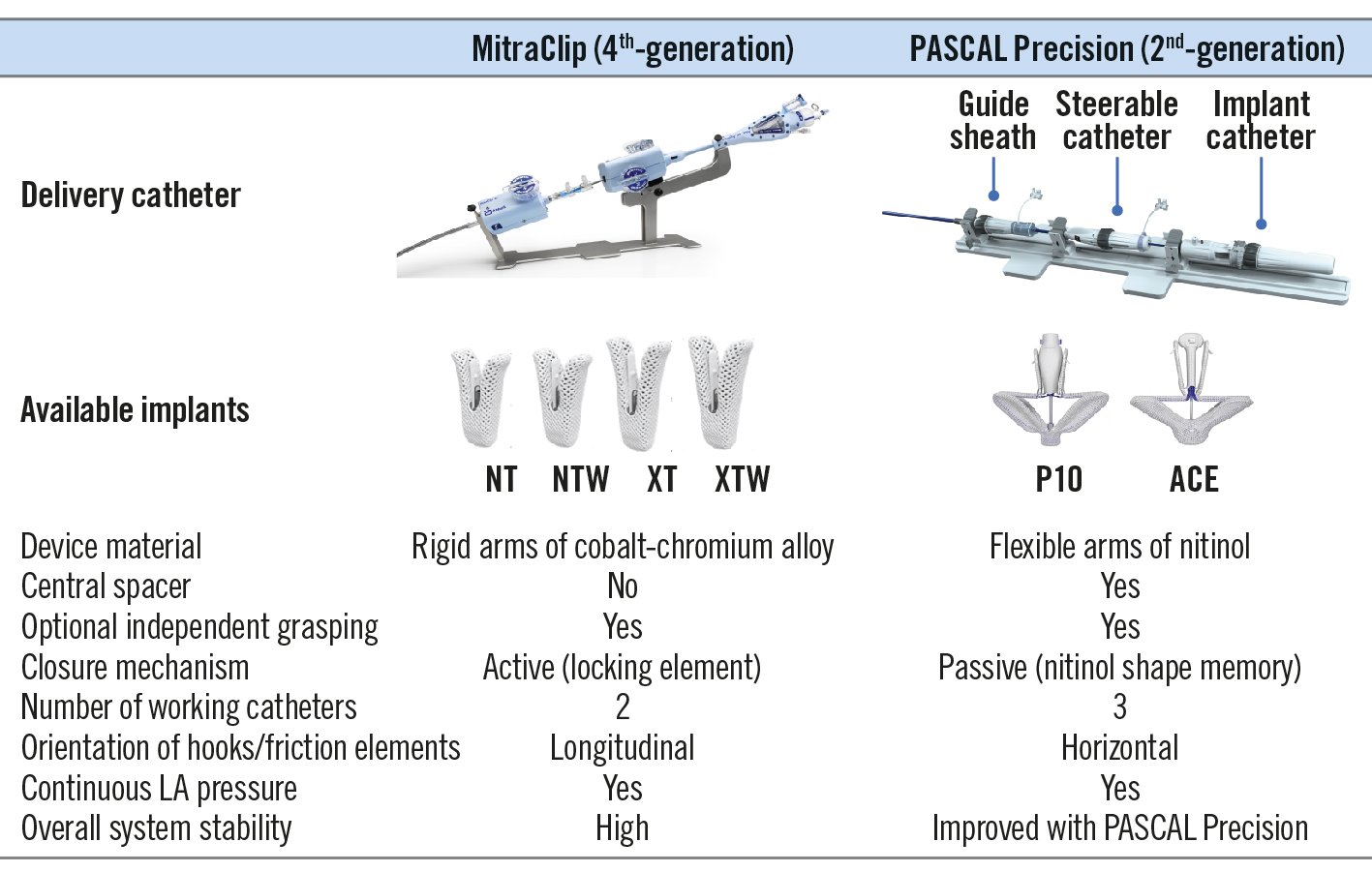
Figure 2. Device overview. Technical specifications of the currently available delivery devices and implants for mitral valve transcatheter edge-to-edge repair. LA: left atrial
The MitraClip system
The MitraClip system is the first transcatheter technology with a CE mark and FDA approval for the treatment of both PMR and SMR13. Since 2020, the fourth-generation MitraClip has been available with 4 different implant sizes (Figure 2)14. Besides the “classic” NT and XT clip sizes (4 mm width; 9 [NT] and 12 [XT] mm arm length), a wider implant size of 6 mm is available with both arm lengths (6 mm width; 9 [NTW] and 12 [XTW] mm arm length). The MitraClip is composed of 2 rigid arms (cobalt-chromium alloy) with flexible nitinol-based “grippers”, which are equipped with 4 (NT/NTW) or 6 (XT/XTW) longitudinally arranged small hooks (“frictional elements"). The longer clip arms (XT/XTW) allow for the treatment of larger coaptation gaps and leaflet flails, beyond the strict anatomic and morphologic EVEREST inclusion criteria15. The expansion of the technique to patients with a more complex anatomy raises concerns about the risk of leaflet injuries and single leaflet device attachment (SLDA) resulting from increased leaflet tension after the grasping of more tissue with the XT/XTW devices, the active locking mechanism, and device stiffness. Leaflet injury due to increased tension forces has been described in different anatomies, including in patients with calcified leaflets15. However, in a structured analysis of the EXPAND registry, the long-arm XTR clip system did not show higher rates of adverse leaflet events compared to the smaller NTR device16. Besides the ability to perform continuous left atrial pressure monitoring through the guiding catheter, the fourth-generation MitraClip system now allows for independent and controlled gripper actuation (CGA) to confirm and/or optimise leaflet grasping.
The PASCAL system
The PASCAL transcatheter MV repair system was first used in 2016 and further investigated within the framework of a compassionate-use cohort including 23 patients with complex anatomical features for M-TEER17. The second generation of the PASCAL system is available and consists of 3 embedded catheters: a 22 Fr steerable guide sheath, a steerable catheter, and an implant catheter with the device preattached at the distal end (Figure 2). This design allows for a high range of motion and facilitates manoeuvrability in the left atrium. The nitinol-based PASCAL P10 implant consists of 2 spring-loaded, curved paddles offering a 26 mm grasping length when open to 180°, as well as 2 clasps (10 mm each) and a central spacer filling the regurgitant orifice. The central spacer is thought to fill parts of the coaptation gap within the main MR jet area, which may reduce the forces on the MV leaflets. The nitinol clasps, which include a horizontal line of small hooks (“retention elements”) at the distal end, can be controlled individually, enabling either simultaneous or independent leaflet capture. A second smaller-size PASCAL Ace has become available, featuring a similar grasping width compared to the PASCAL P10 implant, while the paddles are only 6 mm wide to accommodate smaller anatomies and facilitate multiple implant strategies. Both PASCAL implants allow for independent leaflet grasping enabling either a “leaflet optimisation” or “staged leaflet capturing” technique (see below). The second-generation PASCAL Precision platform was introduced in August 2022, with changes in the catheter system for improving device stability and steerability.
Leaflet optimisation and staged leaflet capture techniques
Besides conventional simultaneous grasping, independent gripper/clasp control enables the frequently used leaflet optimisation technique and, more rarely, the staged capture of the MV leaflets. Leaflet optimisation comes after a simultaneous grasp of both leaflets (Figure 3A-Figure 3C) and consists of reopening the implant arms while the grippers/clasps are kept closed, followed by selective lifting of the chosen gripper/clasp. It allows for the evaluation of the leaflet insertion depth into the respective arm, as well as for independent regrasping of one of the leaflets to achieve a deeper insertion, if required. Successful optimisation frequently results in further MR reduction. Furthermore, small rotations of the device in relation to the line of coaptation are possible to improve device coaxiality.
During staged leaflet capture, a first leaflet is grasped and secured in the device, while the second arm remains in the “capture ready” position with gripper/clasp up (Figure 3D-Figure 3F). Subsequently, the catheter is moved towards the second leaflet and the gripper/clasp is activated when the leaflet is optimally inserted into the device. Both techniques need to be performed with great caution to prevent valve distortion or leaflet injury.
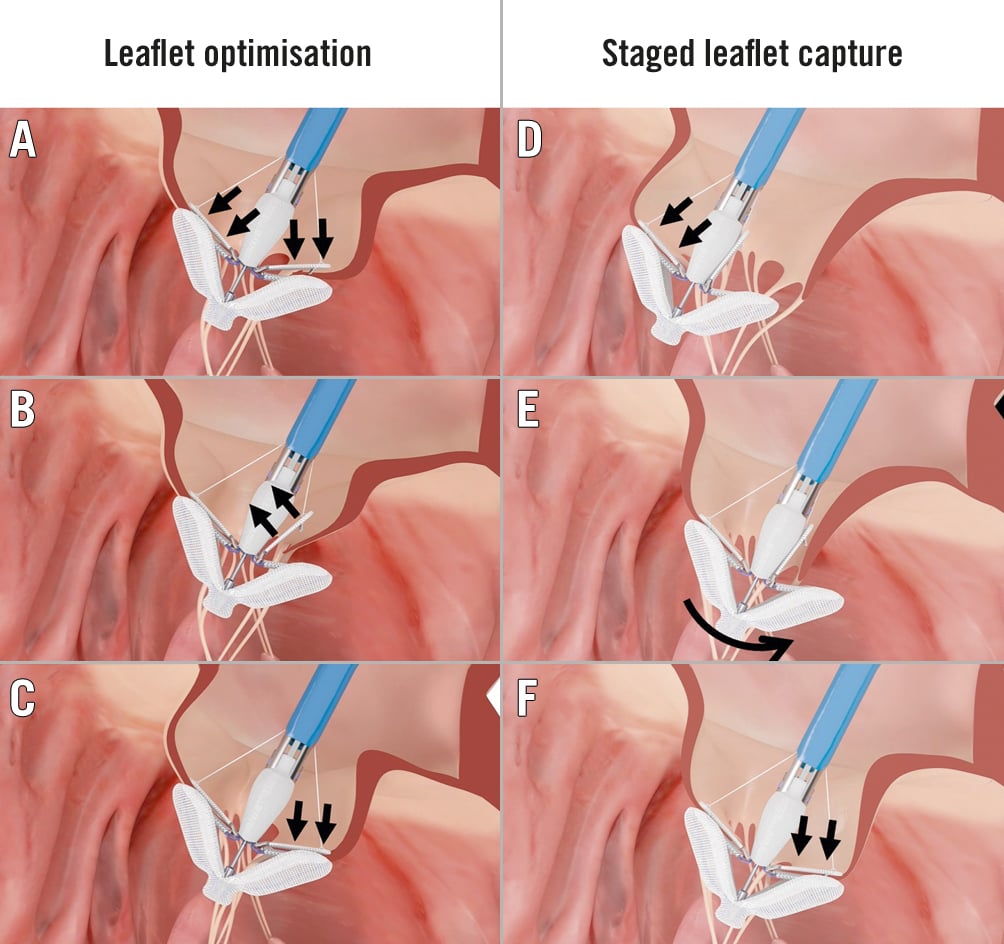
Figure 3. Leaflet optimisation and staged leaflet capture techniques. Leaflet optimisation consists of reopening of the implant arms (A) and selective actuation of the chosen gripper/clasp (B, C). For staged leaflet capture, 1 leaflet is grasped and secured (D), then the catheter is moved towards the other leaflet (E), and the second gripper/clasp is activated (F). Reprinted with permission of Edwards Lifesciences.
Safety of M-TEER
M-TEER is a very safe procedure with a low rate of serious complications despite the high burden of comorbidities of the treated patients. The most frequent complications and their respective occurrence rates are summarised in Table 3.
Specific concerns include the increased risk of leaflet perforation, tear or SLDA in patients with long-standing SMR, as well as in those with short or calcified leaflets. MitraClip embolisation has been described anecdotally in the literature and percutaneous retrieval may be challenging, in particular when larger clips are involved18.
While afterload mismatch may occur in patients with reduced LV dysfunction, it is a rare, temporary phenomenon that can be managed with inotropic drugs and usually does not necessitate mechanical support. Some studies reported a possible negative impact of afterload mismatch on long-term outcomes, which probably reflects the advanced HF stage of patients experiencing this acute complication. Thrombus formation in the left atrium/ventricle has been rarely observed in SMR patients with severely depressed LV function due to the low blood flow conditions19. In such patients, an early and intensified anticoagulation regimen might be indicated.
Table 3. Possible complications after M-TEER.
| Category | Complication | Rate (references) | |
|---|---|---|---|
| Device-related | Structural failure | Single leaflet device attachment | 1.5%-5.1%3334 |
| Device embolisation | 0.05%-0.70%33119 | ||
| Leaflet injury | 0%-2%1415 | ||
| Functional impairment | Residual MR >2+ | 3.4%-17.0%1415 | |
| Transmitral gradient >5 mmHg | Up to 15%15 | ||
| Procedure-related | Access-site vascular | Major vascular complications (significant bleeding, perforation, rupture, dissection) | 1.4%-4.0%120 |
| Cardiac structural damage | Pericardial effusion or tamponade | 0%-0.5%120 | |
| Haemodynamically relevant interatrial septal defect | Not consistently defined | ||
| Bleeding | Severe bleeding requiring blood transfusionPossible bleeding locations: access site, pericardial effusion, gastrointestinal, urinary tract | 0%-17%120 | |
| Thromboembolic | Myocardial infarction | 0%-3%120 | |
| Stroke | 0%-1%120 | ||
| MR: mitral regurgitation; M-TEER: mitral valve transcatheter edge-to-edge repair | |||
Assessment of anatomical complexity
Evaluation of the anatomical complexity represents an essential step of patient and device selection. The likelihood of obtaining an optimal valve repair result with a residual MR ≤1+ and without a clinically relevant increase of the MV inflow gradients must be estimated.
Anatomical inclusion criteria had been already integrated for both PMR and SMR patients in the early EVEREST trial, defining a patient population with a high likelihood for an optimal M-TEER result20. Outside of these EVEREST MV criteria, the complexity of the M-TEER procedure increases, resulting in a lower likelihood of an excellent and durable valve repair, which may subsequently impact outcomes21.
In the EXPAND registry, anatomical complexity was defined using the following criteria: wide coaptation gap (≥15 mm), large flail gap (≥10 mm), jet outside anterior 2/posterior 2 (A2/P2), small mitral valve area (MVA), calcified landing zone, and minimal leaflet tissue. Patients meeting one or several of these criteria were less likely to reach residual MR ≤1+ following M-TEER using the third generation of the MitraClip device. In 2 other studies, the presence of annular and leaflet calcifications, in particular leaflet infiltration of 6 mm or more, MVA <4 cm2, baseline transmitral gradient ≥4 mmHg, and multiple jets have been identified as risk predictors for an increased final transmitral gradient (≥5 mmHg) after M-TEER2223. Identified quantitative predictors of procedural success include the coaptation reserve24, the leaflet-to-annulus index25, and asymmetrical tethering26, which therefore represent additional technical markers of anatomical complexity (Figure 4). Severe calcification in the grasping area, active endocarditis, and haemodynamically relevant mitral stenosis are definite contraindications for M-TEER. However, although technically challenging, patients with failed surgical MV repair have been successfully treated using this procedure27.
Importantly, results following M-TEER have been shown to be highly dependent on centre experience, so that the complexity of the selected cases has to be adapted accordingly (Figure 4)28. Although new device and catheter iterations may help in obtaining optimal M-TEER results29, patients with a complex anatomy might be better referred to high-volume heart valve centres. Indeed, new surgical and interventional techniques, including transcatheter MV replacement instead of M-TEER, may be considered in these patients (Figure 4).
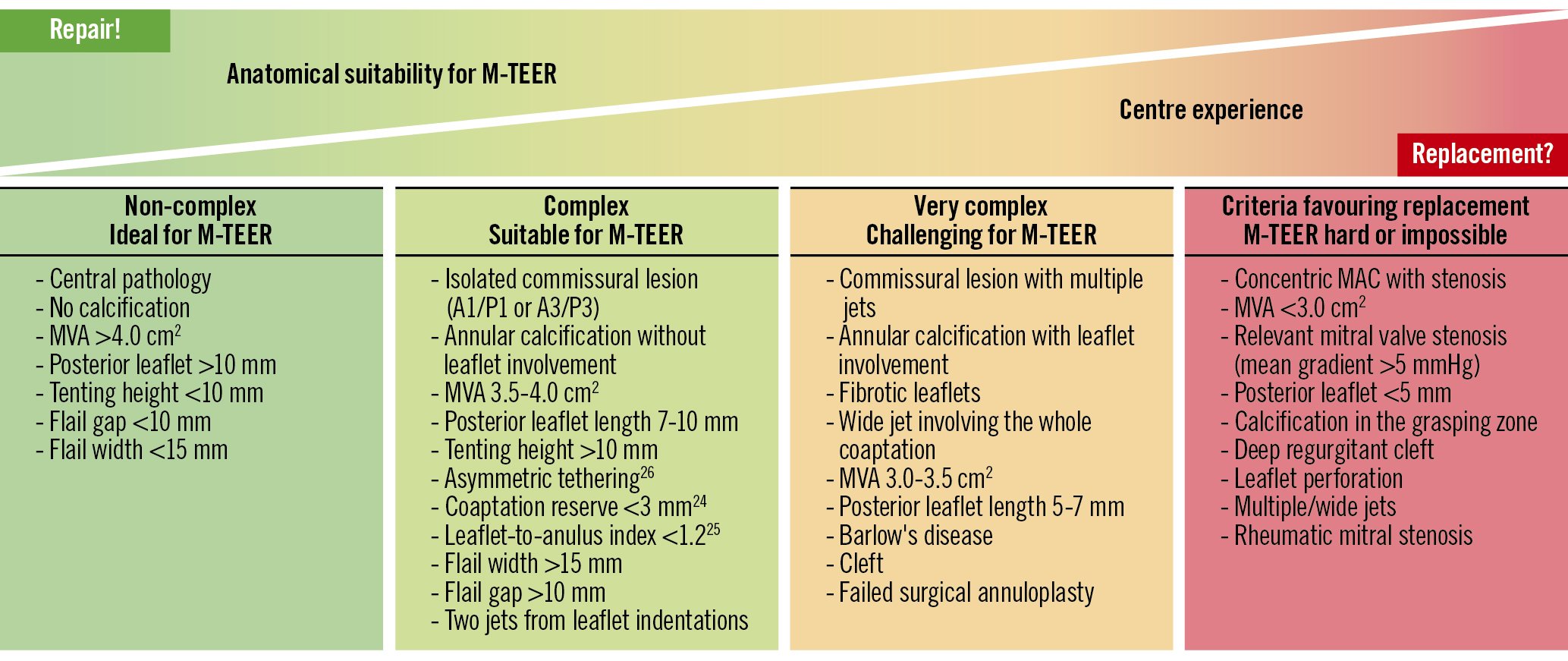
Figure 4. Complexity of valve morphology and centre experience as criteria for mitral valve transcatheter edge-to-edge repair. A1/P1: lateral segments of anterior (A1) and posterior (P3) mitral valve leaflet; A3/P3: medial segments of anterior (A3) and posterior (P3) mitral valve leaflet; MAC: mitral annular calcification; M-TEER: mitral valve transcatheter edge-to-edge repair; MVA: mitral valve area
Treatment of primary MR
To date, surgery represents the standard of care in patients with PMR owing to the excellent long-term efficacy of MV repair in observational studies. MV repair should be preferred over replacement when the valve anatomy is suitable and the perioperative risk is acceptable930. In patients with high or prohibitive surgical risk, M-TEER may be considered by the Heart Team (recommendation class IIb, level of evidence [LoE] B) based on the randomised EVEREST II study and multiple registry results313233. In the American Heart Association/American College of Cardiology (AHA/ACC) guidelines, M-TEER received a class IIa recommendation for selected patients in NYHA Class III or IV34.
The majority of available data on M-TEER in primary MR describe the use of the first and second generations of the MitraClip system. In the EVEREST II trial, where 73% of patients had PMR, similar mortality was observed after 5 years of follow-up for patients treated with the MitraClip device compared to surgery (20.8% vs 26.8%; p=0.4). In terms of efficacy, surgery was superior to the MitraClip procedure in PMR patients (freedom from death, MV surgery, reoperation, and moderate to severe MR at 5 years in 45.5% vs 76.2% of the patients; p<0.001) due to a higher rate of relevant residual MR requiring valve surgery during the first 6 months. In another study, a propensity score-matched comparison in older patients with PMR confirmed the higher long-term recurrence rate after MitraClip implantation (27.0% vs 2.8%; p<0.001), while periprocedural complications and 1-year mortality were lower in the M-TEER cohort35. However, these results are not necessarily transferable to the contemporary landscape, since experience and knowledge about M-TEER were very limited during the EVEREST II enrolment phase, from 2005 to 2008, and older generations of the MitraClip devices were used.
The EXPAND registry specifically evaluated the use of the third-generation MitraClip NTR or XTR in high-risk patients with PMR or mixed aetiology, who represented 40.5% of the 1,041 patients included. Despite the high-risk patient population with a mean age of 79.5±9.4 years, the 30-day event rate was remarkably low (2.4% all-cause mortality, 1.2% stroke, and 0% myocardial infarction), confirming the high safety of the procedure when performed in experienced heart valve centres. A core lab analysis at 30 days revealed a reduction in MR to grade ≤1+ in 86.9% and to grade ≤2+ in 97.3% of cases, which is remarkable considering that 115 (29%) patients presented with complex PMR leaflet pathologies consisting of severely degenerated leaflets or large flail gaps or widths (62.6%), calcification in the landing zone (35.7%), and extremely wide jets (29.6%). In this subgroup with complex MV pathologies, MR reduction to grade ≤1+ at 30-day follow-up still reached 79.4% (MR ≤2+: 96.9%). The ongoing EXPAND G4 registry (ClinicalTrials.gov: NCT04177394) will investigate the safety and performance of the fourth-generation MitraClip system.
In patients with Barlow’s disease, residual MR ≤2+ at discharge was achieved in 76% of the patients compared to 81% in the non-Barlow’s disease group (p=0.40)36. At 3 years of follow-up (completed for 75% of the patients), durability was significantly lower in the Barlow’s disease group (MR ≤2+: 62% vs 80%; p=0.01) resulting in a trend towards a higher rate of HF hospitalisations (17% vs 7% at 3 years; log-rank p=0.07).
The currently enrolling randomised trials, REPAIR MR (Percutaneous MitraClip Device or Surgical Mitral Valve REpair in PAtients With PrImaRy MItral Regurgitation Who Are Candidates for Surgery, ClinicalTrials.gov: NCT04198870), PRIMARY (Percutaneous or Surgical Mitral Valve Repair, ClinicalTrials.gov: NCT05051033), and MITRA-HR (Multicentre Study of MITRACLIP Transcatheter Mitral Valve Repair in Patients With Severe Primary Mitral Regurgitation Eligible for High-risk Surgery, ClinicalTrials.gov: NCT03271762), will aim to compare MitraClip to surgical MV repair in lower- and high-risk patients.
The international early feasibility CLASP Study (Edwards PASCAL TrAnScatheter Mitral Valve RePair System) included 124 patients with PMR (31%) and SMR (69%)37. In this early experience, echocardiographic core lab analysis revealed MR reduction to grade ≤1+ and grade ≤2+ in 77% and 97% at 30 days in the overall cohort, respectively, which were maintained at the 2-year echo follow-up and did not differ between the SMR and PMR cohorts (MR ≤1+ and MR ≤2+ rates of 71% and 100% at 2-year follow-up, respectively). These results were obtained with the exclusive use of the 10 mm wide PASCAL implant (P10); the smaller PASCAL Ace device became available in Europe in 2020. The smaller device might have some advantages when treating primary MR with commissural jets as well as patients with smaller MV areas.
Non-inferiority of the PASCAL system compared to the MitraClip in terms of major adverse events (cardiovascular mortality, stroke, myocardial infarction, new need for renal replacement therapy, severe bleeding and non-elective MV reintervention) and effectiveness (MR ≤2+ at 6 months) has been recently shown in a randomised controlled trial that included PMR patients at prohibitive risk for surgery38.
Treatment of secondary MR
Treatment of SMR patients fulfilling the COAPT criteria
Current recommendations for the treatment of SMR39 mainly refer to the 2 randomised clinical trials (RCT)4041 investigating the role of M-TEER (i.e., MitraClip) in patients with chronic HF on guideline-directed medical therapy (GDMT) compared to GDMT alone. Both studies confirmed the safety and efficacy of MitraClip in reducing MR severity up to 2- and 3-year follow-up4243. However, MITRA-FR failed to demonstrate a prognostic impact of M-TEER on top of GDMT42, while in COAPT, M-TEER reduced the cumulative incidence of HF hospitalisations (primary endpoint; hazard ratio [HR] 0.49, 95% confidence interval [CI]: 0.37-0.63; p<0.0001), and all-cause mortality (powered secondary endpoint; HR 0.67, 95% CI: 0.52-0.85; p<0.001) at 2 and 3 years4243. Many hypotheses have been put forward to explain these diverging results4445. Differences in patient characteristics, the quality of the GDMT scrutinised by an eligibility committee in the COAPT study, the procedural complication rate and M-TEER result durability appear to be the most relevant factors. The COAPT critera are summarised in Figure 5. In the 2021 guidelines, the recommendation for M-TEER has been upgraded (class IIa, LoE B) for selected patients fulfilling the COAPT criteria939 (Figure 1). The recently presented, but not yet published, results of the COAPT postapproval study that included 5,000 patients and reported 1-year clinical outcomes in a contemporary, real-world setting further support the efficacy of M-TEER in SMR.
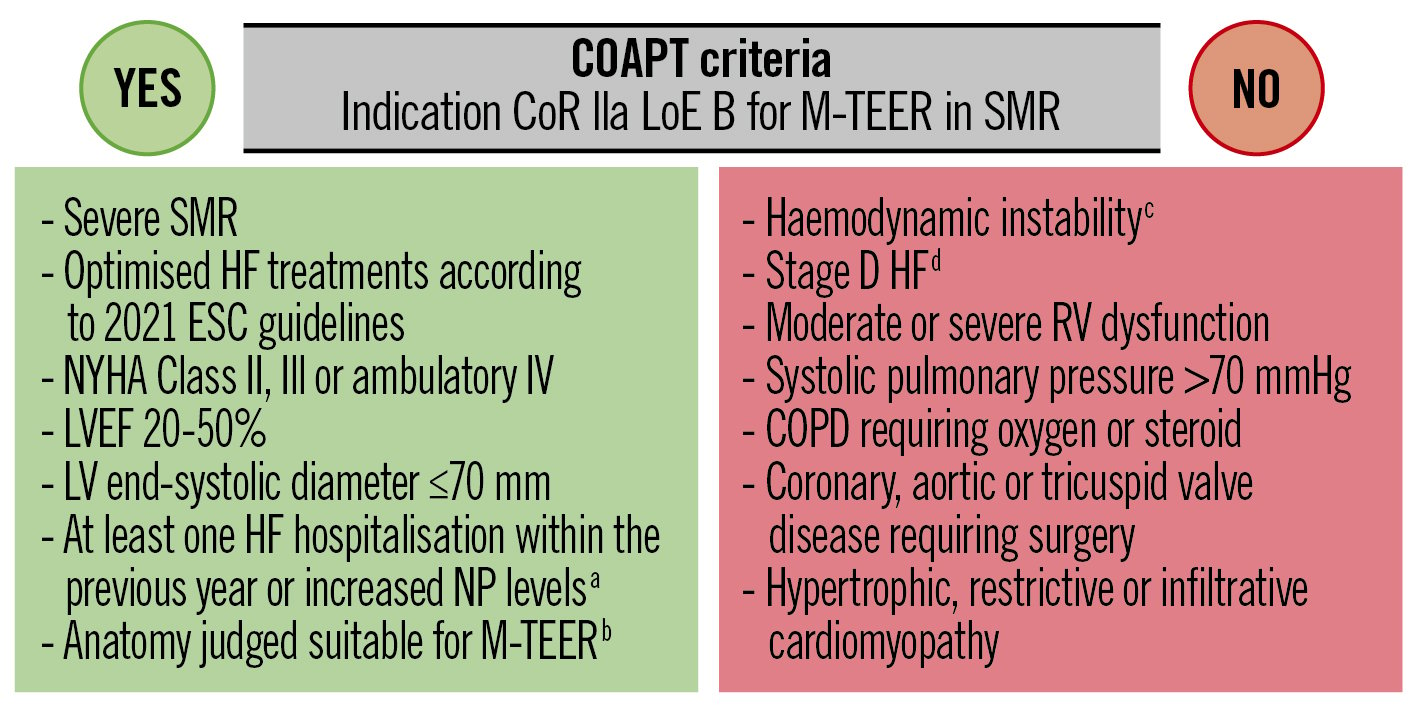
Figure 5. Simplified COAPT criteria Simplified COAPT criteria. Simplified in- and exclusion criteria of the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) study. aBNP ≥300 pg/ml or NT-proBNP ≥1,500 pg/ml. bincluding MVA >4.0 cm2. csystolic pressure ≤90 mmHg, cardiogenic shock or the need for inotropic and/or mechanical supports. dACC/AHA classification. ACC: American College of Cardiology; AHA: American Heart Association; BNP: B-type NP; COPD: chronic obstructive pulmonary disease; CoR: class of recommendation; ESC: European Society of Cardiology; HF: heart failure; LoE: level of evidence; LV: left ventricular; LVEF: left ventricular ejection fraction; M-TEER: mitral valve transcatheter edge-to-edge repair; NP: natriuretic peptide; NYHA: New York Heart Association; RV: right ventricular; SMR: secondary mitral regurgitation
Treatment of SMR beyond the COAPT criteria
The current guidelines also recommend the consideration of M-TEER in patients who do not fulfil the COAPT criteria but in whom M-TEER may improve symptoms and quality of life (recommendation class IIb, LoE C). Patients with chronic SMR who do not fulfil the COAPT criteria include the following 2 main categories: patients with advanced HF and severely reduced left ventricular ejection fraction (LVEF), and patients with atrial SMR and preserved LVEF939 (Figure 1).
In the large retrospective EuroSMR registry, a COAPT-like profile was identified in about 40% of cases and was associated with a lower rate of rehospitalisation and mortality. Interestingly, stratification based on the MITRA-FR criteria failed to predict clinical outcomes46. EuroSMR provides further evidence that both COAPT-eligible and COAPT-ineligible patients experienced an improvement in exercise capacity and quality of life after M-TEER46 and the NYHA class improved independently of the baseline right ventricular function47. Moreover, a similar clinical improvement was observed in patients with EROA above or below a cutoff of 30 mm2 48. In other multicentre studies an improvement in symptoms or quality of life was observed after M-TEER regardless of LVEF or pulmonary pressure4950. Finally, a decrease in pulmonary pressure and NYHA class was observed irrespective of LV reverse remodelling after M-TEER51.
In patients with advanced HF, the role of M-TEER as a bridge strategy to LVAD implantation or HTx was evaluated in a multicentre cohort of 119 patients. At a median follow-up of 532 days, 13% of patients died, 44% underwent LVAD implantation or HTx and 26% were removed from the waiting list due to improvement of their clinical and haemodynamic conditions52. In another small retrospective study, M-TEER reduced the pulmonary vascular resistance in patients with end-stage HF and severe SMR, potentially increasing their eligibility for HTx53.
Although the best treatment modality of patients with atrial SMR is not yet known, emerging evidence shows atrial reverse remodelling and an improvement of symptoms after M-TEER54. In EuroSMR, the prevalence of atrial SMR was 7.8%, and the outcomes were encouraging in patients considered at high risk for surgery, particularly if advanced HF symptoms (e.g., NYHA Class IV) or RV dysfunction were absent55.
Predictors of outcomes after M-TEER in SMR
Several parameters predict an increased risk of clinical events (i.e., mortality and/or HF hospitalisation) in SMR patients undergoing M-TEER. Advanced symptoms (NYHA Class III and/or IV) were found to be associated with poor outcomes in large observational registries5657, and a 28% higher risk of mortality or HF hospitalisation per 1 NYHA class increase was observed in COAPT at 2 years58. The independent prognostic role of LVEF was reported in the registries and the COAPT trial125759, whereas the role of LV dimensions is more controversial12. Chronic atrial fibrillation may entertain and worsen SMR and has a negative impact on outcomes60.
Many parameters used to assess the right ventricular function, as well as tricuspid regurgitation, also have a prognostic relevance in patients undergoing M-TEER6162. More recently, the right ventricular-pulmonary arterial coupling index was identified as a powerful predictor of adverse events in both the EuroSMR registry47 and the COAPT trial63. In addition, chronic obstructive pulmonary disease (COPD)64, chronic kidney disease65, and pulmonary hypertension5066 were independently associated with poor prognosis in both M-TEER registries and COAPT. Importantly, the prognostic benefit of M-TEER in combination with GDMT versus GDMT alone was attenuated in patients with COPD compared to patients without chronic lung disease (interaction p-value=0.04 for mortality)64.
Treatment optimisation before M-TEER in SMR
GDMT optimisation and implementation of cardiac resynchronisation therapy (if indicated) before M-TEER are essential pillars of SMR treatment since they may enable LV reverse remodelling and decrease MR severity67. In a recent observational study, 42% of patients with chronic HF and MR 2+/3+ experienced an improvement of MR severity after GDMT initiation and optimisation. However, persistence of MR 2+ or 3+ was associated with an almost 2-fold increased risk of mortality or HF hospitalisation at long-term follow-up68, indicating that the goal of GDMT optimisation should be to achieve MR 1+. Active restoration of a stable sinus rhythm through cardioversion and/or ablation has been associated with a decrease in SMR severity in a small prospective study69. However, a minority of patients with relevant SMR respond to the above-mentioned treatments and the magnitude of MR reduction is lower compared to M-TEER70. Finally, the hypothesis that successful M-TEER may facilitate GDMT uptitration and therefore impact survival outcomes requires further investigation.
Before M-TEER, SMR patients should be appropriately treated with diuretics to reach a euvolemic status, taking into account that intraprocedural vasodilatation and hypotension induced by general anaesthesia may lead to an underestimation of SMR severity. Thus, both hypovolemic and hypervolemic conditions should be avoided. Neurohormonal drugs should be maintained during the periprocedural period. In patients with severe LV dysfunction and/or large coaptation gaps, a preparation with inotropic drugs and/or an intra-aortic balloon pump has been proposed71 but are not part of daily clinical practice.
Procedural tips and tricks
Device selection
The main factors to be considered for device selection are displayed in Table 4 and Figure 6. Careful evaluation of the disease mechanism, baseline MVA, mean transmitral gradient, and anatomical complexity using 3D echocardiography represents the essential initial steps towards appropriate device selection.
The MVA should ideally be measured using multiplanar reconstruction on specifically acquired high resolution 3D volumes of the MV. Available data concerning previous device generations provide guidance regarding the MVA reduction after device implantation: the implantation of a PASCAL P10 device has been shown to reduce the MVA by about 47%. The use of rigid implants with extended arms, e.g., MitraClip XT or XTW, is expected to have a higher impact on the baseline MVA. Accordingly, the MVA reduction using the NTR and the XTR implants was 52% and 57%, respectively72. Importantly, the MVA reduction also depends on the device localisation along the line of coaptation, with the maximal reduction occurring in the A2/P2 position (“hot zone”) and minimal MVA reduction occurring after commissural placement72.
Jet localisation and treatment strategy are 2 additional key factors influencing device selection. Indeed, in patients with distinct jets, in whom the implantation of 2 distant clips is expected, a higher baseline MVA of about 6 cm2 is required to avoid creating a relevant MV stenosis72. In patients with large flail gaps or wide prolapses, devices with extended arms (XT, XTW or PASCAL) seem to be more effective in reducing MR, in particular if multiple implants are used for stabilisation73. When a multiple clip strategy is anticipated, the use of the PASCAL P10 should be avoided since the matching of 2 implants may not be optimal due to the concave shape of the paddles. For the treatment of isolated commissural lesions, implants with small arms (e.g., NT/NTW) and stable steering should be preferred.
A detailed assessment of leaflet tissue quality (length and thickness) is of paramount importance. The presence of annular calcifications with leaflet infiltration has been identified as a predictor of an increased transmitral gradient after M-TEER and should motivate the use of smaller/more flexible devices2223.
In SMR patients with a short and/or thin tethered posterior leaflet, devices with extended arms, such as the MitraClip XT and XTW, should be avoided in order to prevent SLDA or leaflet injury. The use of the PASCAL devices appears less problematic due to the flexible nitinol design and horizontal orientation of the grasping elements, in particular in the presence of a short posterior leaflet, because the grasping force is applied at the leaflet base (“hinge point” with the mitral annulus).
Table 4. Recommendations for “preferred” device selection in PMR and SMR with respect to anatomical features.
| Anatomical features | NT | XT | NTW | XTW | PASCAL | PASCAL ACE | |
|---|---|---|---|---|---|---|---|
| Length of the mobile leaflet in the grasping zone |
<9 mm | ✓ | ✓ | ✓ | ✓ | ||
| >9 mm | ✓ | ✓ | ✓ | ✓ | |||
| Broad gap size | ✓ | ✓ | ✓ | ||||
| Small MVA (<4.5 cm2) | ✓ | ✓ | ✓ | ||||
| Thin leaflet structure | ✓ | ✓ | ✓ | ✓ | |||
| Commissural jet | ✓ | ✓ | ✓ | ||||
| Barlow’s disease | ✓ | ✓ | ✓ | ✓ | |||
| MVA: mitral valve area; PMR: primary mitral regurgitation; SMR: secondary mitral regurgitation | |||||||
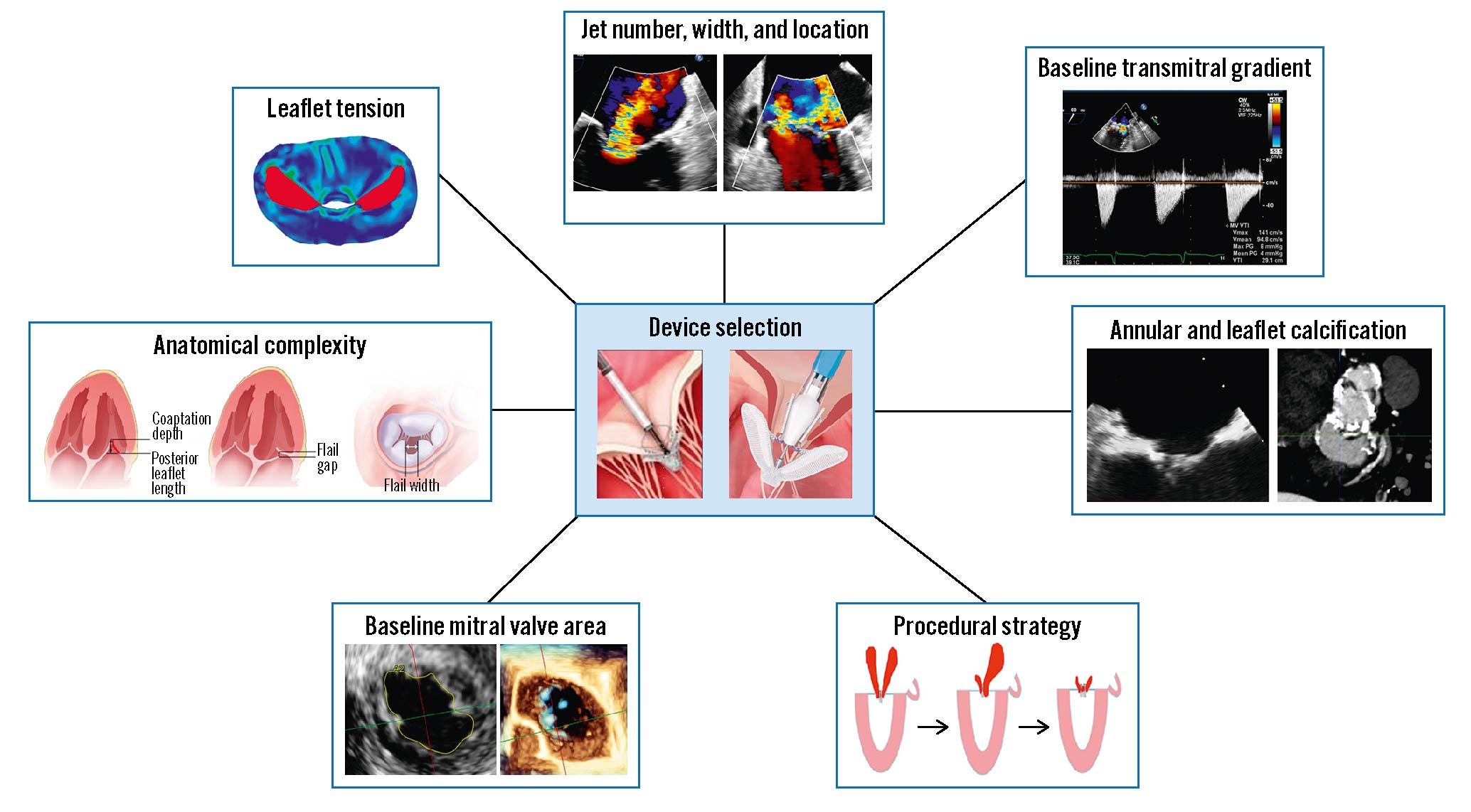
Figure 6. Criteria to be considered for device selection. Device selection criteria include characteristics of the regurgitant jet, baseline transmitral gradient, annular and leaflet calcification, procedural strategy, mitral valve area, anatomic complexity, and leaflet tension.
Intraprocedural imaging and guiding
Procedural guiding of an M-TEER procedure requires dedicated skills and should be performed by appropriately trained interventional imagers. Position documents describing the requirements for an interventional imager have been recently published7475. During the procedure, fluoroscopy as well as echocardiography are used for guidance. To improve communication between the interventionalist and the interventional echocardiographer, fusion imaging can be used. This technique facilitates anatomical orientation through simultaneous visualisation of catheters and soft tissue.
An assessment of the MV anatomy and regurgitation should be repeated before starting the procedure, since SMR is a dynamic condition, highly influenced by volume status and haemodynamics. In addition to traditional 3D rendering (Figure 7A) and colour Doppler (Figure 7B), advanced 3D imaging techniques including GlassVue (Figure 7C) and TrueVue (Figure 7D) (both Philips) may provide additional information regarding leaflet tissue quality and the exact origin of the jet. Using multiplanar reconstruction, the MVA is measured at the beginning of each procedure to refine device selection and plan the treatment strategy (Figure 7E).
An ultrasound-guided puncture of the femoral vein below the inguinal band might reduce retroperitoneal bleeding complications and arterial mispunctures. The transseptal puncture is performed starting from a bicaval view (90-120°) (Figure 7F) using an X-plane (red line) to localise and adjust the position of the needle tip in the antero-posterior plane. Using fusion technology, these 2D views can be shown on top of the fluoroscopy. In the more recent releases, even a full 3D segmentation of the heart can be made in which the optimal transseptal puncture zone can be identified. Following successful septum crossing, the position of the curved stiff wire in the left pulmonary veins (usually the left upper) or left atrium needs to be confirmed. Fusion imaging is useful to guide both this step and catheter steering towards the mitral plane (Figure 7G-Figure 7H), avoiding interaction with the left pulmonary veins and the left atrial appendage. Device orientation is typically performed using a 3D view from the left atrium. The “cardiologist’s” 3D view of the MV with orientation of the aorta at 6 o’clock allows corresponding device movement to the intercommissural 2D view and simplifies the procedure when compared to the traditional “surgeon’s” 3D view of the MV (Figure 8). Adjustment of the X-ray gantry angulation after confirming a perpendicular device orientation in 3D echo is helpful for controlling and maintaining an identical device orientation during the grasping process by fluoroscopy (Figure 9).
For valve crossing and grasping, consistently oriented standard views are necessary as shown in Figure 7I. An X-plane (red line) is typically placed on an intercommissural view (50-70°) in order to obtain a left ventricular outflow tract (LVOT) view (140-160°) of the area of interest showing both leaflets in the A2/P2 segment separately. Simultaneous visualisation of both planes allows for the correction of the trajectory of the catheter, which should be maintained in the LV to facilitate leaflet grasping and avoid valve distortion. Pulling/pushing the whole assembly, as well as flexing/unflexing the steerable catheter, can be visualised in the intercommissural view and will displace the catheter towards the medial and lateral clips, respectively. While looking at the LVOT view, a clockwise rotation of the catheter will move the implant towards the posterior and a counterclockwise rotation towards the anterior. Flexing the guide sheath will allow the correction of an “aorta-hugger” position or help to lose height, while a posterior rotation will help to gain height. For commissural pathologies, the “intercommissural” plane needs to be adjusted to 30-60° for A3/P3 and to 60-90° for A1/P1 to be parallel to the line of coaptation in the respective mitral segment. The optimal views can more easily be created using multiplanar reconstructions from a 3D view of the MV.
After leaflet grasping, the residual MR should be evaluated in a systematic manner using a multiparametric approach including pulmonary vein flow and left atrial pressure. Leaflet insertion should be carefully assessed and quantified before device release76. Measurement of the transmitral gradient by continuous wave (CW) Doppler or its estimation using pressure half-time is not sufficient to exclude significant MV stenosis, since these parameters depend on several haemodynamic factors, including flow conditions (pre- and afterload), heart rate and diastolic filling, that may be altered during general anaesthesia. Therefore, additional planimetric measurements of each neo-orifice should be performed, ideally with 3D TOE, or alternatively with 2D TOE in the transgastric short-axis view77.
In patients with strict contraindications for TOE or insufficient imaging quality, 4D intracardiac echocardiography may represent an emerging alternative (Figure 10). For better image quality, an ICE catheter has to be introduced into the left atrium through a dilated septostomy that will also accommodate the M-TEER system (Figure 10A). After an assessment of baseline MR (Figure 10B), leaflet grasping is performed using a reconstructed biplanar view (Figure 10C, Figure 10D). Multiple clip strategies are possible to further reduce MR (Figure 10E). In the case of remaining relevant interatrial shunt (Figure 10F), closure of the atrial septal defect can be easily guided from the right atrium (Figure 10G).
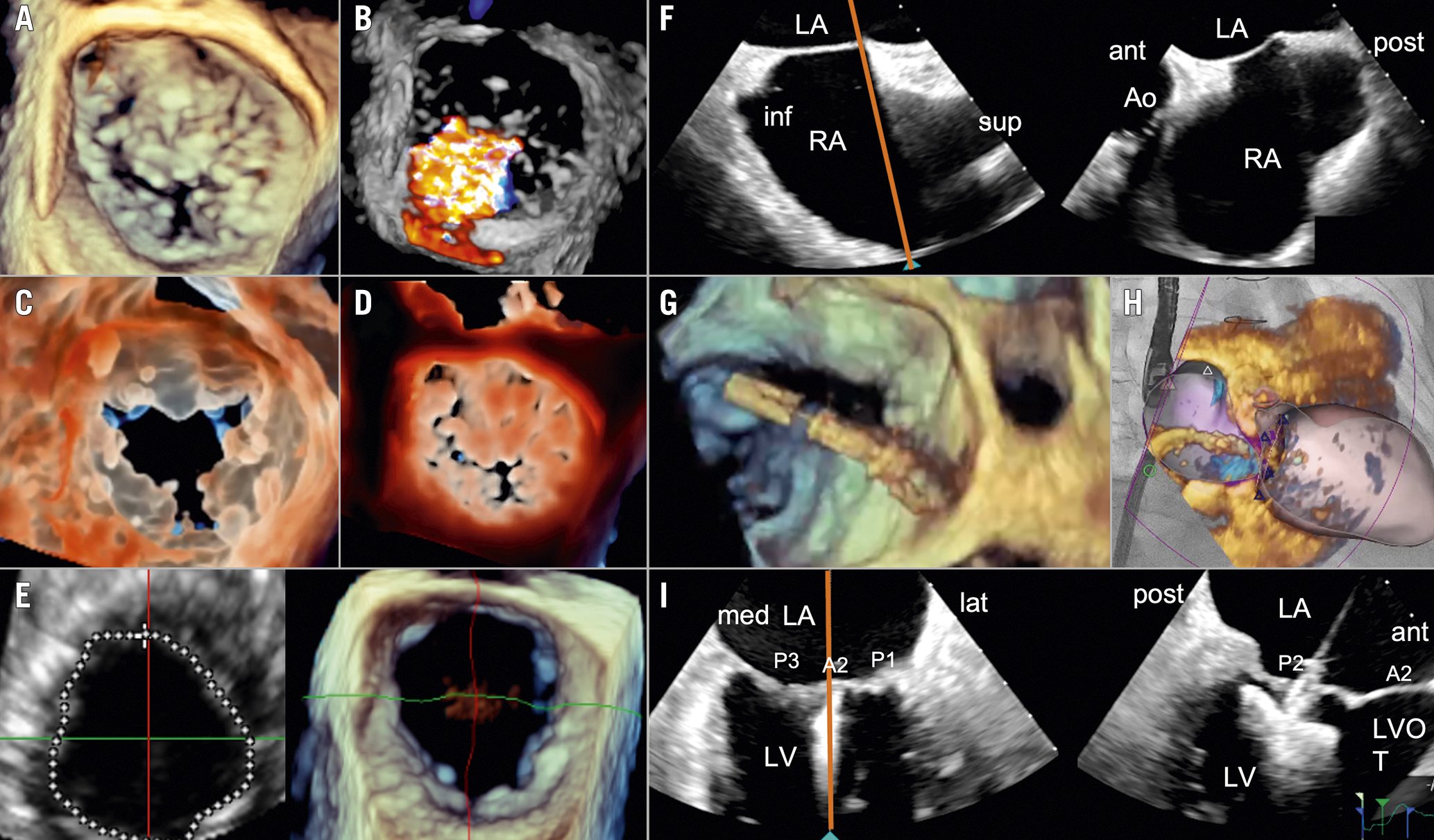
Figure 7. Imaging techniques for visualisation of the mitral valve. Imaging techniques include traditional 3D rendering (A), colour Doppler (B), and 3D imaging approaches such as GlassVue (C) and TrueVue (D). The mitral valve area is measured at the beginning of the procedure to refine device selection and plan the treatment strategy (E). The transseptal puncture is performed starting from a bicaval view using an X-plane to localise and adjust the position of the needle tip in the antero-posterior plane (F). Fusion imaging is useful during catheter steering towards the mitral plane (G, H). Valve crossing and grasping is usually performed on an intercommissural view using an X-plane (I). A2: middle scallop of the anterior leaflet of the mitral valve; ant: anterior; Ao: aorta; inf: inferior; LA: left atrium; lat: lateral; LV: left ventricle; LVOT: left ventricular outflow tract; med: medial; P1, P2, P3: lateral, middle, and medial scallops of the posterior leaflet of the mitral valve; post: posterior; RA: right atrium; sup: superior
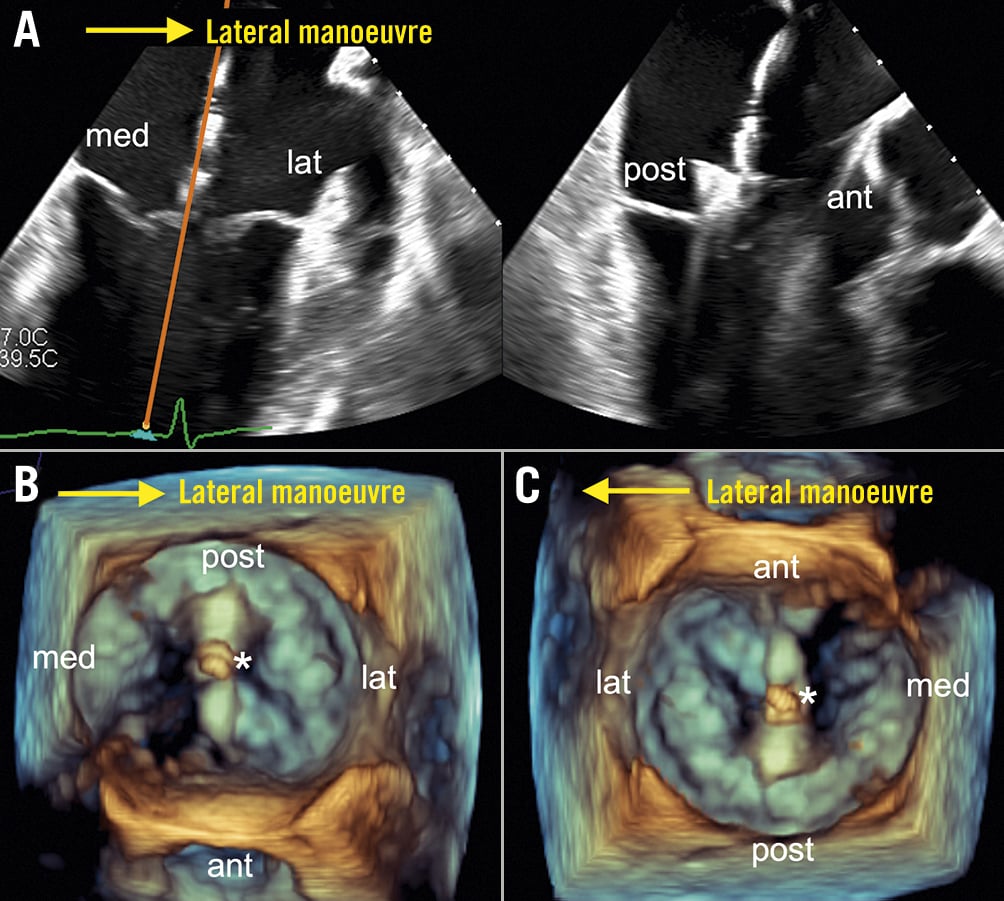
Figure 8. Cardiologist's 3D view of the mitral valve during transcatheter edge-to-edge repair. In the intercommisural 2D view of the mitral valve, lateral manoeuvring of the device (*, here: PASCAL Ace implant) moves the device from left to right (A). The “cardiologist's” 3D view of the mitral valve simplifies device steering since a lateral manoeuvre leads to corresponding movement of the device from left to right (B). In contrast, the traditional “surgeon's” view does not correspond to the intercommisural 2D view of the mitral valve and lateral manoeuvring of the device leads to movement from right to left (C). Therefore, the “cardiologist's” view might be the preferred 3D echo orientation during mitral valve transcatheter edge-to-edge repair. ant: anterior; lat: lateral; med: medial; post: posterior
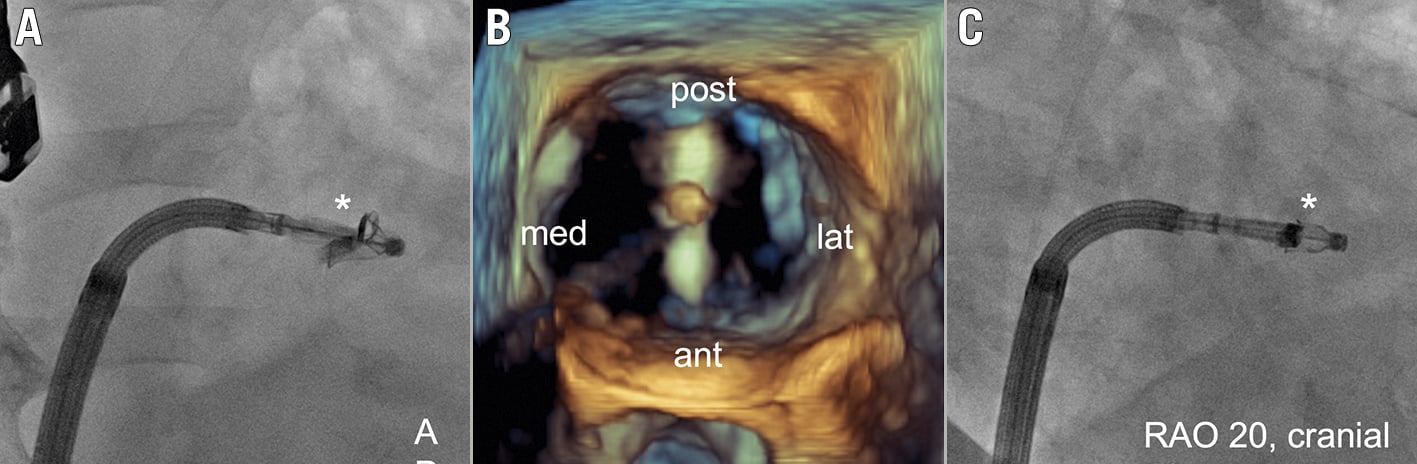
Figure 9. Fluoroscopic device orientation. The orientation of a PASCAL Ace implant (*) is shown by fluoroscopy in an anterior-posterior (AP) projection (A). After correct orientation of the device as confirmed by 3D echo (B), the X-ray gantry should be adjusted until the device arms are superimposed and “disappear” (here: right anterior oblique [RAO] 20, cranial 10 projection) to support the control of the device orientation by fluoroscopy during subsequent leaflet grasping (C). ant: anterior; lat: lateral; med: medial; post: posterior
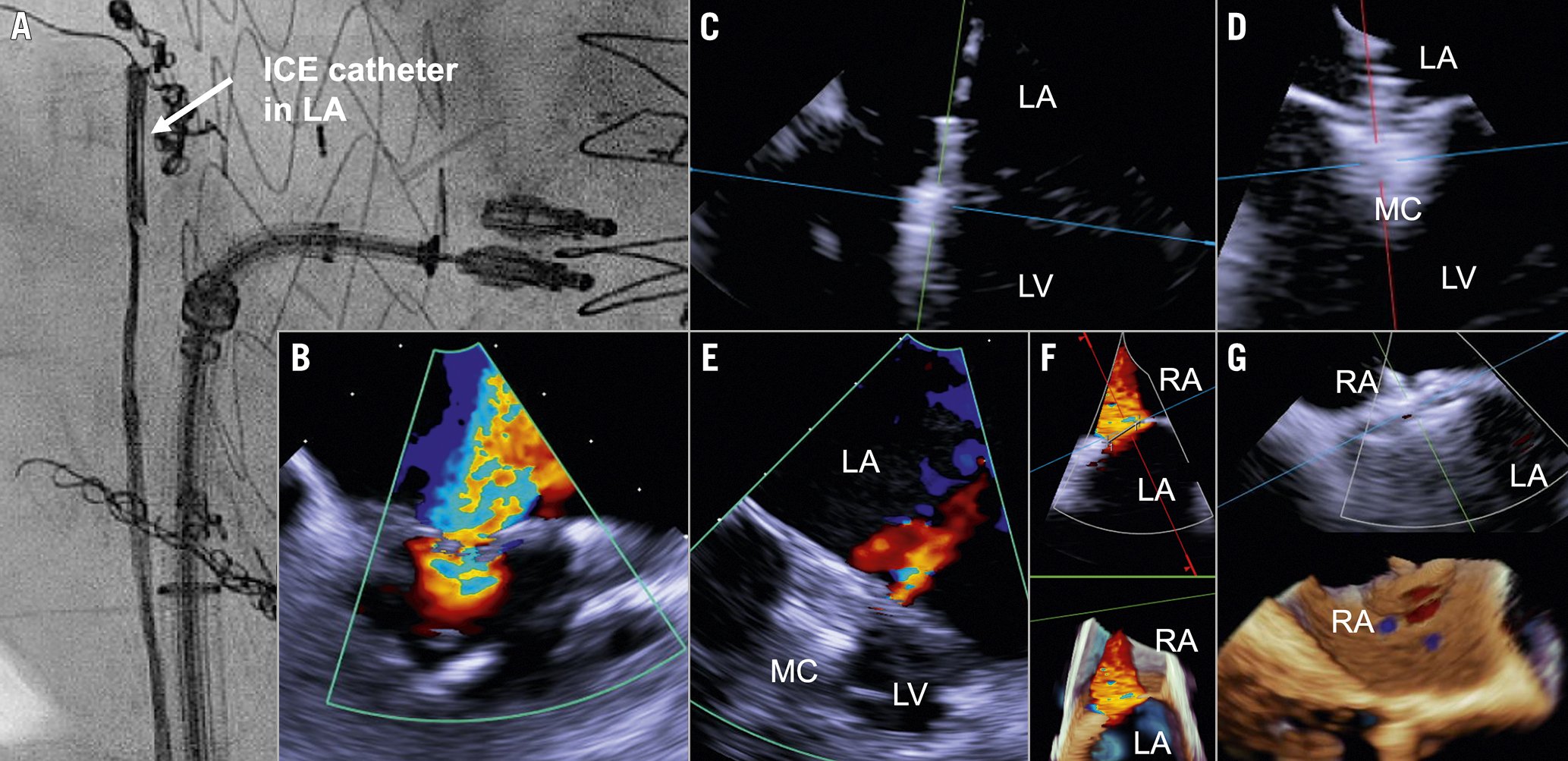
Figure 10. 4D intracardiac echocardiography. Placement of an ICE catheter in the left atrium allows for 4D intracardiac echocardiography (A) to assess MR (B), guide leaflet grasping (C, D), and support multiple clip treatment strategies (E). In case of remaining relevant interatrial shunt (F), closure of the atrial septal defect can be easily guided from the right atrium (G). ICE: intracardiac echocardiography; LA: left atrium; LV: left ventricle; MC: MitraClip; MR: mitral regurgitation; RA: right atrium
M-TEER optimisation
During the procedure, haemodynamic conditions as close as possible to those observed in the awake state should be reproduced. The transseptal puncture should be performed taking into account that the line of coaptation is usually lower in SMR than in PMR. Posterior rotation of the sheath and needle will help to gain height over the mitral plane. Bending (or, more rarely, straightening) the needle helps to establish contact with the fossa ovalis in a distorted anatomy. In the case of difficult crossing (thickened or floppy septum), the use of electrocautery applied directly on the conventional needle or by means of a dedicated device may be useful7879. Alternatively, the stiff end of a coronary percutaneous coronary intervention wire can be advanced into the needle to cross the septum. The optimal distance between transseptal access and the coaptation point is about 4.0-4.5 cm.
Achieving an optimal procedural result (residual MR ≤1+) was associated with a lower rate of mortality and HF hospitalisation compared to an acceptable result (residual MR ≤2+) in a large cohort of PMR patients undergoing M-TEER32. In SMR, optimal MR reduction is still debated. In the COAPT trial, no outcome difference was found between patients with residual MR grade 0/1+ and 2+, while in registry studies a positive impact of lower residual MR was observed808182. In challenging cases, MR reduction should be balanced against the increase of the transmitral gradient (>5 mmHg), which has been associated with worse outcomes83. While a final MVA <1.5cm2 should be avoided, a higher cut-off of ≤1.94 cm2 was already associated with a blunted reduction of pulmonary artery hypertension and a higher incidence of adverse events at 2 years in a retrospective analysis84.
The implantation of more than 1 device may be considered according to residual MR and gradients, in particular in patients with PMR and a large flail/prolapse gap or width. In this case, the first device is usually positioned more medially and the second device lateral to the first device. In the presence of a large coaptation gap, a zipping-clip technique might be considered85.
A cleft-like indentation, especially between P1-P2 and P2-P3 scallops, can be observed in patients with severe leaflet tethering or annular dilatation. The presence of such lesions can cause residual jets after M-TEER, particularly if the leaflet tissue is thinned toward the indentation. If the main jet arises from the indentation, a 2-device strategy with the lateral clip oriented slightly more clockwise than the line of coaptation and the medial one oriented slightly more counterclockwise can be attempted (convergent clips technique)86.
In patients with multiple segmental prolapse (Barlow’s disease), the anchor technique, which involves positioning a clip in a region adjacent to the main jet, can reduce the coaptation gap and allow for more effective MR treatment73. The application of positive end-expiratory pressure and breath-hold after forced expirations increases intrathoracic pressure leading to a reduction of the LV preload and MV coaptation gap87. In general, an intraprocedural reduction of respiratory tidal volumes results in minimising the respiratory-associated heart motion, which allows for stabilisation of the TOE image plane as well as precise leaflet grasping.
Two techniques have been used for closure of femoral vein access: manual compression plus “figure-eight” (or “Z-”) suture, followed by a compression bandage for, e.g., 12 hours, or a suture-mediated closure device (e.g., ProGlide; Abbott), followed by a shorter-duration compression bandage. While both techniques appear to be of comparable safety and efficacy, the latter may allow for an earlier patient mobilisation8889.
Haemodynamic monitoring
Invasive haemodynamic monitoring is recommended during M-TEER procedures to evaluate immediate efficacy. Haemodynamic changes, in particular the mean left atrial pressure, have been linked to clinical outcomes90. This includes the measurement of the left atrial pressure and, in particular, the left atrial v-wave. Additionally, cardiac output can be measured using the thermodilution or Fick method. Invasive pressure measurements may be more reliable than echocardiographic colour Doppler to estimate MR improvement and possibly also clinical response, especially in complex MV anatomies91. A decrease in left atrial pressure after correction of SMR may be less pronounced than in PMR due to concomitant impaired diastolic filling.
Management of recurrent MR and SLDA
In the event of relevant residual or recurrent MR, the indication for MV surgery or re-intervention needs to be discussed by the Heart Team. TOE must usually be repeated to understand the underlying pathology and identify the appropriate leaflet portion for additional device placement, as well as to estimate the risk of the patient developing of relevant mitral stenosis.
Alternative interventional approaches for the treatment of significant interclip or paraclip residual MR have been described in case series with limited safety data. These include the implantation of an Amplatzer vascular plug (Abbott), originally designed for embolisation of the peripheral vasculature92, or implantation of an expanded polytetrafluoroethylene double-disk occluder, originally designed for closure of atrial septal defects93. Electrosurgical laceration and stabilisation of the clip (ELASTA-clip) followed by transapical MV replacement (e.g., implantation of a Tendyne bioprosthesis [Abbott]) represents an emerging option for selected patients with residual/recurrent MR or development of mitral stenosis after M-TEER94.
Implant failure due to SLDA or loss of leaflet insertion occurs in 3.5% of patients according to a large multicentre registry and is associated with higher in-hospital (8.2%) and longer-term mortality (29.3% at a median follow-up of 163 days)95. Although the majority of these patients are treated conservatively, redo M-TEER is feasible and may be preferred over surgery in anatomically suitable PMR patients, as well as in SMR patients with reduced LVEF, in whom surgical outcomes are dismal96.
Major unsolved questions and grey areas
Heart valve centres and case volume
Due to the complex anatomic changes leading to MR, the requirement for optimal echocardiographic imaging and the coordinated interaction of interventionalists and imagers, transcatheter MV repair is considered one of the most challenging interventional procedures. To achieve optimal outcomes, e.g., residual MR ≤1+, M-TEER should be performed in dedicated heart valve centres with an experienced Heart Team, because centres with low M-TEER volumes may experience higher complication rates28. Accordingly, a minimum number of M-TEER procedures per hospital and investigator is necessary to ensure high-quality patient outcomes97. While the inflection points in the learning curves for procedural time, procedural success and procedural complications occur after approximately 50 cases, continued improvements were observed up to 200 cases. Importantly, this does not only apply to operator experience but, rather, to the whole team, in particular the interventional imagers98. Furthermore, with the evolving field of new transcatheter treatment options including, e.g., valve annuloplasty, chordal replacement and valve replacement, high-volume heart valve centres will have the possibility to select the most appropriate technique for the individual patient out of this rapidly expanding portfolio. Future research and guideline recommendations should further address the association between procedural case volumes and outcomes after M-TEER.
M-TEER in multivalvular heart disease
Patients with multivalvular heart disease represent an underrecognised and less well-studied patient population. Guideline recommendations for this patient group are limited. The most common valvular heart disease combinations are (I) mitral regurgitation and aortic stenosis, and (II) mitral and tricuspid regurgitation. The value of an experienced Heart Team is of particular importance for the clinical decision-making in such patients. Due to the interactions between different valve lesions, several potential diagnostic pitfalls need to be considered99.
The combination of MR and severe aortic stenosis is frequently observed. In the Placement of Aortic Transcatheter Valve (PARTNER) trial, moderate-to-severe MR was reported in 20% of patients at high risk undergoing aortic valve intervention100. The following diagnostic caveats should be considered: (a) in the presence of aortic stenosis, a high intraventricular pressure may result in higher mitral regurgitant volumes, whereas mitral EROA is less affected; (b) the presence of MR may favour a low-flow, low-gradient state; and (c) the high-velocity MR jet may be mistaken for the aortic stenosis jet. Besides the severity of each valve disorder, MR aetiology – primary versus secondary – has a relevant impact on the sequence of the treatment. In patients with SMR ≥2+, several studies demonstrated MR improvement after aortic valve replacement101102. Therefore, in transcatheter aortic valve implantation (TAVI) candidates with SMR ≥2+, TAVI should be performed first and MR severity re-evaluated at short-term follow-up (e.g., at 3 months)103. In patients with severe PMR, the likelihood of improvement is lower; therefore, sequential TAVI and M-TEER should be planned. If performed first, M-TEER may result in the aggravation of the coexisting aortic stenosis due to the increased forward flow.
Patients undergoing M-TEER suffer from concomitant TR ≥2+ or ≥3+ in 56.3% and 18.9% of the cases, respectively (Table 5)104105. In a small retrospective study, an incremental increase of the 6-minute walking distance and a reduction of N-terminal pro-brain natriuretic peptide (NT-proBNP) resulted in fewer rehospitalisations, with sequential treatment of both valves compared to isolated treatment of the MV106. The TRAMI registry demonstrated increased in-hospital, 30-day and 1-year mortality in patients with combined severe MR and TR undergoing isolated M-TEER57. Since M-TEER results in reduced pulmonary congestion with lower pulmonary artery pressure, a post-procedural reduction of secondary TR severity can be expected. In fact, the prevalence of TR ≥2+ is reduced by 21% at follow-up (from 56.3% to 44.7%) (Table 5), indicating only a modest impact of M-TEER on TR severity.
While simultaneous TR repair is recommended in patients undergoing left-sided valve surgery (class Ib), the role of simultaneous or sequential transcatheter treatment of MR and TR is less clear. Comparing outcomes from the TRAMI and TriValve registries, a significant reduction in mortality was observed in patients with concomitant edge-to-edge repair of both valves compared to treating the MV only107, but prospective randomised data addressing this important question are lacking.
Table 5. M-TEER registries.
| TRAMI | Zürich | GRASP | Hamburg | Mainz | Italy/Spain | Munich | Summary | |
|---|---|---|---|---|---|---|---|---|
| Publication | EuroIntervention 2017121 | EuroIntervention 2016122 | EHJ CVI 2014105 | EuroIntervention 2017123 | Clin Res Cardiol 2021124 | Eur J Heart Fail 2022125 | Unpublished | |
| No of patients | 766 pts | 119 pts | 146 pts | 139 pts | 606 pts | 503 pts | 602 pts | 2,881 pts |
| TR ≥3+ | 106/766 | 26/119 | - | 11/139 | 129/560 | 137/503 | 99/602 | 18.9% (508/2,689) |
| TR ≥2+ | 432/766 | 66/119 | 47/146 | 81/139 | 319/560 | 344/503 | 306/602 | 56.3% (1,595/2,835) |
| TR ≥2+ at FU |
- | 35/67 | 16/143 | 52/133 | 191/426 | 287/503 | 135/331 | 44.7% (716/1,603) |
| Selection of European M-TEER (mitral valve transcatheter edge-to-edge repair) registries. In total, 2,881 patients (pts) were reported. Tricuspid regurgitation (TR) was evaluated before M-TEER in 2,835 patients and 56.3% of patients demonstrated moderate TR ≥2+. Severe TR ≥3+ was reported in 18.6% of cases (508 of 2,689 patients). After M-TEER, the rate of TR ≥2+ was reduced from 56.3% to 44.7%. FU: follow-up; No: number; Ref: reference | ||||||||
The role of atrial fibrillation therapy in the context of SMR
MR, atrial fibrillation, and LVEF have a complex interplay. The incidence of AF depends, among other factors, on the severity and duration of MR108. Consequently, AF is associated with worse outcomes in both patients with PMR108 and SMR60. Furthermore, AF itself increases the MV annular area, which worsens MR, and creates a vicious cycle. Interestingly, M-TEER compared with GDMT alone was associated with a lower risk of stroke in patients with HF history and AF109. This might suggest that M-TEER reduces AF burden in HF patients leading to a lower risk of thromboembolic complications. The transition from sinus rhythm to AF is associated with a sudden loss of atrial contraction and leads to higher filling pressures. In particular, HF patients benefit from regaining atrial contraction, and therefore early AF ablation or antiarrhythmic therapy with or without M-TEER might be considered in patients with AF and MR. However, the best approaches and timing for interventions to reduce MR on the one hand and restore sinus rhythm on the other are still inadequately understood.
M-TEER in cardiogenic shock and acute MR
Haemodynamic instability and cardiogenic shock were exclusion criteria in previous M-TEER trials and registries, so that outcome data for such patients are scarce. Small observational studies suggest that MR patients with advanced LV dysfunction and cardiogenic shock may benefit from M-TEER as a rescue and bridging strategy110111.
Acute MR due to papillary muscle rupture following myocardial infarction is a rare condition which is often accompanied by cardiogenic shock and pulmonary oedema. M-TEER has been used successfully as an alternative to surgical intervention in selected high-risk patients112113. Interestingly, in patients with moderate-to-severe MR following myocardial infarction, M-TEER was associated with lower in-hospital and 1-year mortality rates when compared to surgical MV repair or replacement114.
Patients with cardiogenic shock undergoing successful M-TEER seem to have more favourable outcomes at short-term follow-up compared to those receiving an unsuccessful procedure, suggesting a potential benefit of MR reduction in this setting that needs to be confirmed in randomised trials115.
Risk stratification in M-TEER
A variety of scores have been developed to predict and improve outcomes in patients undergoing M-TEER. In 2016, a multimodality assessment score of intraprocedural MR using echocardiography, angiography and left atrial haemodynamics (called MitraScore) has been associated with improved MR reduction at discharge116. Whether the application of this multimodality MitraScore will improve 1-year survival after M-TEER is currently being studied in the large prospective MitraPro registry (DRKS00012288).
In addition, the MITRALITY score and a second score, which has also been termed “MitraScore”, were developed to predict 1-year mortality after M-TEER117118. Interestingly, there is only limited overlap between the variables used in the different scores, indicating that the prediction of mortality after M-TEER remains challenging and not fully understood so far. Thus, future research is necessary to optimally predict outcomes, as well as futility. Such models might be enhanced if they address PMR and SMR patient cohorts independently.
Conclusions
To date, M-TEER is the most important transcatheter treatment strategy of the MV. Technical device improvements and increasing procedural experience have consolidated M-TEER as an effective option in selected patients with a wide range of anatomies. Consequently, M-TEER is currently recognised as an alternative to surgery in the latest American and European guidelines. It can be expected that M-TEER will permanently remain in the transcatheter MV treatment portfolio, which will be further expanded by new percutaneous replacement solutions during the upcoming years.
Acknowledgements
We would like to thank Dr. Nicolas Brugger, MD, Bern University Hospital, for acquiring and providing the echocardiographic images included in Figures 6, 7, and 10.
Conflict of interest statement
J. Hausleiter has received speaker honoraria and research support from Edwards Lifesciences outside the submitted work. T.J. Stocker has received compensation for travel expenses from Edwards Lifesciences. M. Adamo has received speaker honoraria from Abbott Vascular and Medtronic outside the submitted work. N. Karam has received consultant fees from Abbott Vascular outside the submitted work. M.J. Swaans reports personal fees from Abbott Vascular, Boston Scientific, Philips/Volcano, Edwards Lifesciences, and BioVentrix, outside the submitted work. F. Praz has received compensation for travel expenses from Abbott Vascular, Edwards Lifesciences, and Polares Medical.
Supplementary data
To read the full content of this article, please download the PDF.

