Transcatheter edge-to-edge repair (TEER) is now a class IIa (1) or IIb (2) recommendation for patients with high surgical risk and patients with severe primary mitral regurgitation (MR), and a IIa recommendation for patients with severe symptomatic secondary MR fulfilling specific anatomic and haemodynamic criteria1,2. Although outcomes have been favourable, the rate of clip-related complications, in particular single leaflet device attachment (SLDA) and leaflet injury, continue to raise concerns.
Complications of mitral TEER have varied with the implantation learning curve and device iterations. A recent report of the first four years of commercial TEER implantation using the Manufacturer and User Facility Device Experience (MAUDE) database recorded 200 death reports and 1,666 injury reports, with 8% of injuries requiring additional procedures or surgical intervention3. Although the study did not calculate rates of injury, the most common reported injuries included SLDA, clip detachment, and entanglement in chordae tendineae. Both operator and institutional volume are associated with procedural success rates, procedure time, and procedural complications4,5. The ACCESS-EU registry (first-generation device) reported a 4.8% SLDA rate6, whereas the large (n=2,952) “real-world” report from the STS/ACC TVT Registry (first-generation device) reported an SLDA rate of only 1.5%7. Early reports from the MitraClip EXPAND Study (ClinicalTrials.gov Identifier: NCT03502811), a post-market observational study of the third generation of the device, reported a 4% SLDA rate in the first 107 patients, with all device detachments associated with leaflet tearing and use of the MitraClip® XTR device (Abbott Laboratories, Abbott Park, IL, USA)8. A single-site report comparing MitraClip® NTR and XTR confirmed higher leaflet injury associated with the larger device (XTR 14.6% vs NTR 1.7%, p=0.012) with an overall leaflet tear rate of 4.4% and an SLDA rate of 2.7%9.
Although the Mitral Valve Academic Research Consortium (MVARC) provides a list of device-related complications that should be reported in clinical trials10, there is no strict definition of each complication, making comparisons between site reports and clinical trials more difficult. Therefore, Asch et al11, published in this issue of the Journal, should be commended on this first attempt towards standardising the definitions of these complications.
The authors11 convened a multidisciplinary expert panel consisting of interventional echocardiographers, interventional cardiologists and cardiovascular surgeons, to perform a patient-level analysis of the EXPAND database (1,041 patients, third-generation devices) to reach a consensus on definitions of leaflet complications. Baseline MR aetiology in evaluable transthoracic echocardiograms (TTEs) were: 46% primary MR, 49% secondary MR, and 5% mixed. The site-reported overall incidence of device-related complications was 3.4% (n=35) with leaflet injury in 1.1% and SLDA in 2.3%. The adjudication committee disagreed with 15 of these cases and reported an overall incidence of device-related complications of 2.0% (n=20) with leaflet injury in 0.4% (n=4) and SLDA 1.7%. However, as sites may have access to other images or the real-time feedback from their imagers, there may have been justification for classifying the non-adjudicated cases as “real” complications. Although somewhat speculative, a worst-case scenario for leaflet injury (Figure 1A) could include all 11 site-reported injuries, as well as the 5 SLDA cases seen by the site, and yet be “ruled out” by the committee (possible “partial gripping”), resulting in a potential leaflet injury rate of 1.5%. The number of adjudicated SLDA clips was 22 (18 confirmed, plus 2 cases with double SLDAs, and 2 cases occurring with leaflet injury); however, 5 SLDA cases could not be confirmed due to lack of any images and, if we assume these could be real, it suggests a total of 27 SLDA cases (Figure 1B) and a probable SLDA rate of 2.6%. The total device-related leaflet complication rate would therefore be 4.1% (43/1,041), which appears closer to real-world commercial data9.
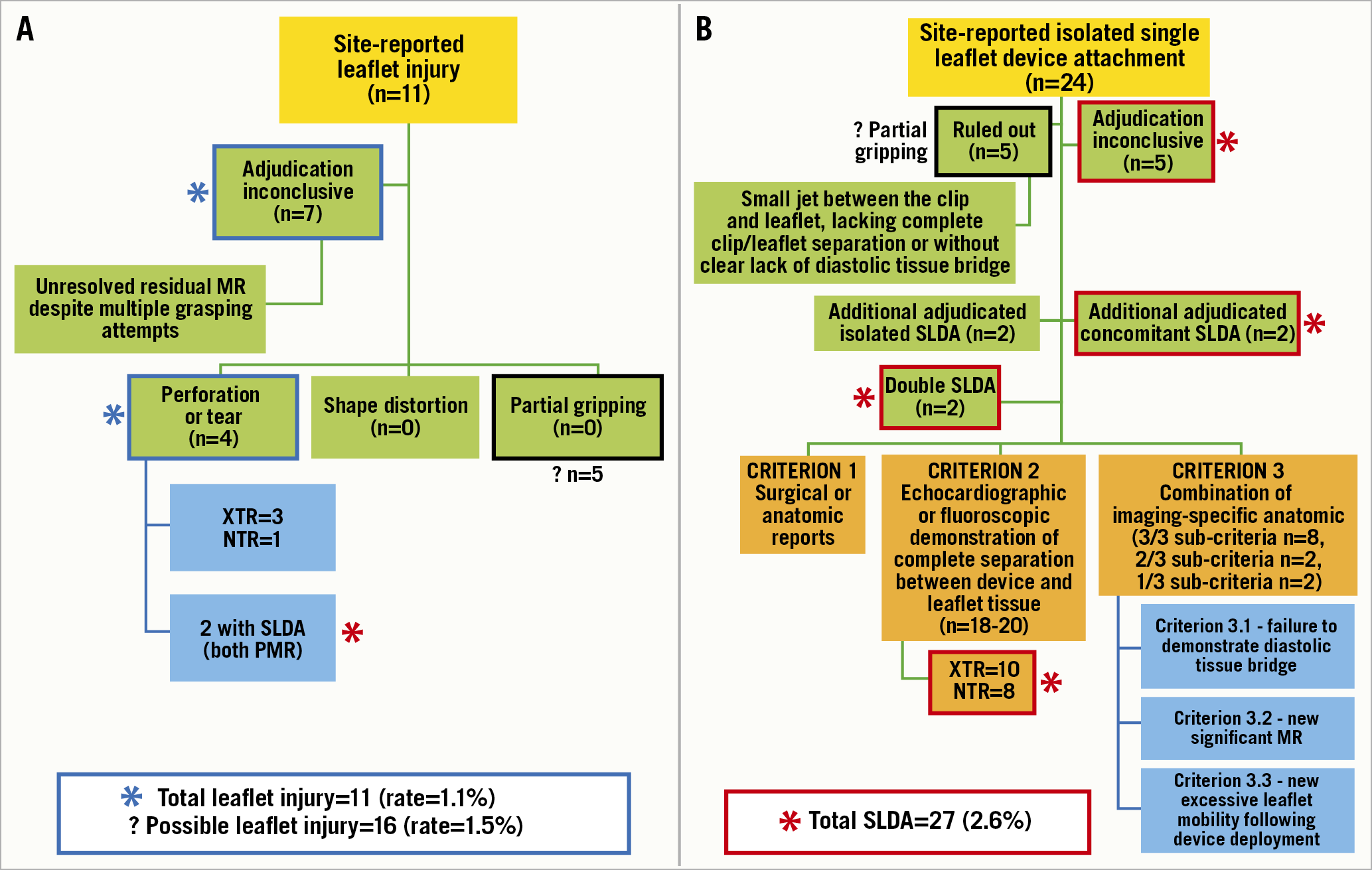
Figure 1. Transcatheter edge-to-edge repair device-related complications reported in the EXPAND registry. A) The adjudication results of patients with leaflet injury; the blue asterisk indicates patients who should be included as having likely leaflet injury, including the inconclusive, site-reported events. In addition, five site-reported single leaflet device attachment (SLDA) patients did not have this complication when adjudicated by the committee; however it seems likely that some abnormality was seen (most likely partial gripping), and thus the possible total leaflet injury patients could be as high as 16 patients. B) The adjudication results of patients with SLDA; the red asterisk indicates patients that should be part of the total (including the five patients who did not have imaging submitted for adjudication, two additional detached clips from patients with double SLDA, and two from the leaflet injury patients).
There are conclusions from this report that thus require some equipoise. First, the anatomic criteria for leaflet injury listed in the methods include: tear, perforation (these are combined later in the analysis), leaflet shape distortion and partial gripping. These last two leaflet complications were never identified by the committee, although as discussed above, partial gripping may have been underdiagnosed. Leaflet distortion may be a superfluous category: if the leaflet is distorted or twisted, injury and/or SLDA are more likely and clinically more important. A distorted leaflet without these other complications may have little clinical relevance. Second, of the three major criteria to define SLDA, all patients fulfilled one: echocardiographic or fluoroscopic demonstration of complete separation between the device and leaflet tissue (criterion 2), and the other criteria appear unnecessary. Third, the seven cases which the committee did not agree to label as leaflet injury continued to have significant MR following multiple grasping attempts; the assumption of leaflet injury (or perhaps partial gripping or chordal entanglement) would be reasonable. Fourth, sub-criterion 3.1 and the major criterion 2 (Figure 1B) are essentially the same (complete separation between the device and tissue, and absence of a tissue bridge). The other sub-criteria used to support a finding of SLDA are non-specific: sub-criterion 3.2 (significant MR through the device/leaflet interface) may represent leaflet injury or cleft and not SLDA; and sub-criterion 3.3 (new excessive leaflet mobility) may represent chordal entrapment/rupture or partial leaflet insertion.
The suggested low rates of complications for both NTR and XTR in the complete EXPAND series is undoubtedly encouraging although numbers are small (13 patients receiving an XTR vs 9 patients with NTR) and, together with single site reports, suggest a possibly greater risk of leaflet injury with the XTR compared to the NTR device. The rate of complications should lower with design improvements that reduce chordal entanglement and enable independent leaflet grasping. In addition, given the high mortality reported with surgical repair following failed TEER implantation12, non-surgical solutions for device removal, allowing for the subsequent delivery of a transcatheter replacement devices, may become an attractive solution13 in the ~3-4% of patients with device-related valve complications.
Importantly, Asch et al hope to help differentiate leaflet injury events from inadequate leaflet insertion and SLDA, and to provide guidance for the accurate diagnosis of leaflet events with a TEER device. However, the inability to adjudicate complications, most often in the setting of inadequate imaging, highlights the need to generate a comprehensive post-TEER imaging protocol for an accurate assessment of device function. These protocols should include when and how to use non-standard views and/or advanced imaging tools (i.e., three-dimensional imaging, transoesophageal echocardiography or multimodality imaging) to make an accurate diagnosis of leaflet injury and SLDA.
Thus, simplified definitions and specific imaging protocols to diagnose leaflet injury and SLDA could improve our understanding of complication rates. It is reassuring that the TEER procedure for mitral regurgitation is relatively safe and associated with high efficacy. However, as surgical intervention for correction of leaflet injury is required in up to 8% of patients and is associated with high morbidity and mortality, improvements in device design or intraprocedural imaging should aim to reduce these complications further.
Conflict of interest statement
R. Hahn reports speaker fees from Abbott Structural, Baylis Medical, and Edwards Lifesciences; institutional educational and consulting contracts for which she receives no direct compensation with Abbott Structural, Boston Scientific, Edwards Lifesciences and Medtronic; equity with Navigate; and is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation.
Supplementary data
To read the full content of this article, please download the PDF.

