Abstract
Background: In the context of primary mitral regurgitation (PMR), the selection of patients for transcatheter edge-to-edge repair (TEER) does not include a systematic assessment of PMR-associated cardiac remodelling.
Aims: We aimed to investigate the epidemiology and prognostic significance of different phenotypes of extra-mitral valve (MV) cardiac involvement in a large series of patients with PMR referred for TEER.
Methods: The study included 654 patients from the multicentre Italian GIOTTO registry, stratified into groups according to extra-mitral valve (MV) cardiac involvement. The primary endpoint was all-cause death at 2-year follow-up.
Results: Patients with no cardiac involvement (NI; n=58), left heart involvement (LHI; n=343) and right heart involvement (RHI; n=253) were analysed. Acute technical success was achieved in 98% of patients. Kaplan-Meier curve analysis revealed significantly worse survival in patients with LHI and RHI (p=0.041). On multivariate Cox regression analysis, extra-MV cardiac involvement, haemoglobin level and technical success were independent predictors of the primary endpoint occurrence.
Conclusions: Grading cardiac involvement may help refine risk stratification, since at least 1 group of extra-MV cardiac involvement represents in itself a negative predictor of midterm outcome.
Introduction
Mitral regurgitation (MR) is one of the most prevalent valvular heart diseases in Western countries, with a significant impact on morbidity and mortality1. As the burden of significant MR increases consistently with ageing2 and the elderly often have multiple comorbidities affecting their operative risk, up to 50% of patients with severe symptomatic MR are not referred for surgery3.
Percutaneous techniques and, in particular, transcatheter edge-to-edge repair (TEER) have been proposed as feasible and effective therapeutic options for patients not suitable for mitral valve (MV) surgery4. The TEER MitraClip system (Abbott) is the device with the largest body of scientific evidence to support its use5.
Currently, in the context of primary MR (PMR), the selection of patients for TEER is exclusively based on the technical feasibility of the transcatheter approach and surgical risk assessment, without a systematic evaluation of other elements − such as cardiac remodelling associated with MR − that may have a relevant prognostic impact. Thus, the population of patients with PMR who are most likely to benefit from this treatment has yet to be identified.
Hence, the aim of this study was to investigate the epidemiology and prognostic significance of different phenotypes of extra-MV cardiac involvement in a large series of patients with PMR referred for TEER with the MitraClip system and to assess the predictors of clinical outcome. Accordingly, we sought to provide a useful tool to improve the risk stratification of candidates for TEER in this subset of patients.
Methods
STUDY POPULATION
The Italian Society of Interventional Cardiology (GIse) registry Of Transcatheter treatment of mitral valve regurgitaTiOn (GIOTTO) is an ongoing single-arm, multicentre, prospective registry of patients with significant symptomatic MR who have undergone TEER with the MitraClip system in Italian hospitals6.
The present analysis included patients with moderate-to-severe (3+) or severe (4+) PMR treated between February 2016 and May 2020. Registry inclusion and exclusion criteria, echocardiographic selection and protocols, together with data collection and follow-up scheduling have been previously detailed7. Briefly, preprocedural transthoracic and transoesophageal echocardiography were performed to assess the mechanism of regurgitation and morphological suitability for MitraClip implantation. PMR was identified based on the main mechanism of regurgitation, whether this was due to prolapse, leaflet flail or restricted leaflet motion. MR and tricuspid regurgitation severity were graded according to current guidelines by means of a multiparametric approach4. Left ventricular (LV) end-diastolic and end-systolic diameters were evaluated from the parasternal long-axis view. LV end-diastolic and end-systolic volumes were assessed using the Simpson biplane method in the apical 2- and 4-chamber views and indexed to body surface area. The LV ejection fraction was then calculated. The maximum left atrial diameter was derived from the parasternal long-axis view in end-systole8. Systolic pulmonary artery pressure (sPAP) was estimated by the sum of the transtricuspid pressure gradient, calculated with the simplified Bernoulli equation, and right atrial pressure, derived from the diameter and inspiratory collapse of the inferior vena cava9. Tricuspid annular plane systolic excursion (TAPSE), derived from M-mode imaging of the right ventricle in the apical 4-chamber view, was used to quantify right ventricular function8. Patients without an exhaustive echocardiographic examination, including the aforementioned parameters, were not considered for statistical analysis. Details regarding TEER with the MitraClip system have been formerly described10. All patients included in the study signed a written informed consent after receiving an oral and written explanation of the issues concerning the procedure, data collection and subsequent analysis. The investigation conforms to the principles outlined in the Declaration of Helsinki.
DEFINITION OF EXTRA-MV CARDIAC INVOLVEMENT
On the basis of their baseline transthoracic echocardiography, patients were classified into 3 groups according to the extent of extra-MV cardiac involvement (Central illustration): namely, the no extra-MV cardiac involvement (NI) group; the left heart involvement (LHI) group, including patients with LV or left atrial dilation/dysfunction − defined as LV end-systolic diameter ≥40 mm or LV end-systolic volume index ≥30 mL/m2 or LV ejection fraction ≤60%, or maximum left atrial diameter ≥55 mm or history of atrial fibrillation, respectively; and the right heart involvement (RHI) group, considering as inclusion criteria pulmonary circulation or tricuspid valve involvement, defined as sPAP >50 mmHg or tricuspid regurgitation grade >2+, or right ventricle to pulmonary circulation uncoupling, defined as a TAPSE/sPAP ratio <0.307 mm/mmHg. These criteria, with their associated cut-off values, were selected based on current recommendations for the management of valvular heart disease4 and evidence from previous analyses1112. From most to least extent of extra-MV cardiac involvement, patients were assigned to groups in the following hierarchical order: RHI, LHI and NI.
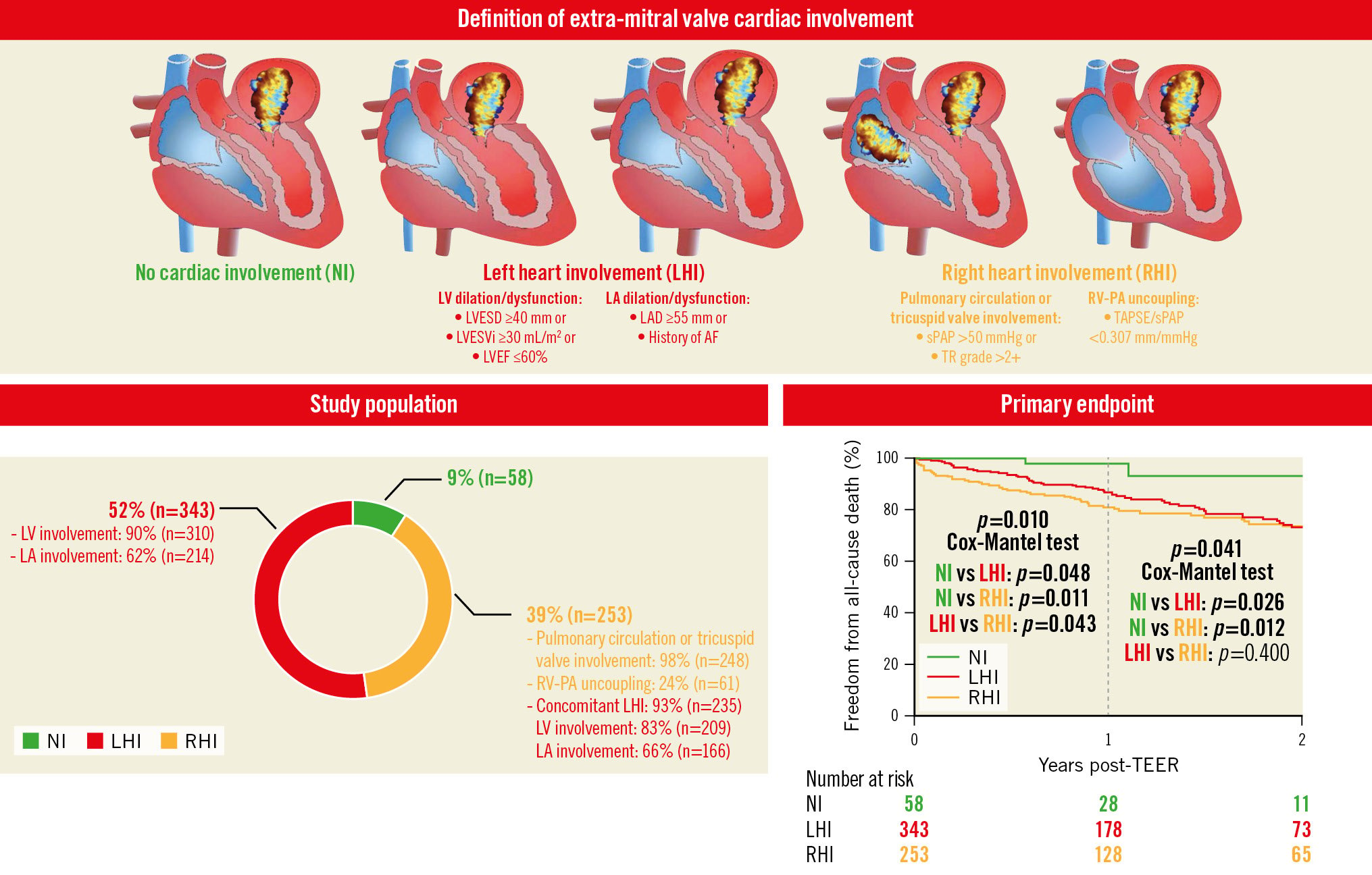
Central illustration. Definition and prognostic significance of extra-mitral valve cardiac involvement. Patient classification based on baseline transthoracic echocardiography characteristics mirroring the extent of extra-mitral valve (MV) cardiac involvement. Distribution of the study cohort according to the extent of extra-MV cardiac involvement. Kaplan-Meier survival estimates for the occurrence of the primary endpoint. P-values are bold only when statistically significant (<0.05). AF: atrial fibrillation; HF: heart failure; LA: left atrium; LAD: left atrial diameter; LV: left ventricle; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESVi: left ventricular end-systolic volume index; NI: no extra-mitral valve involvement; RV: right ventricle; RV-PA: right ventricular to pulmonary circulation; sPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion; TEER: transcatheter edge-to-edge repair; TR: tricuspid regurgitation
STUDY ENDPOINTS
As regards acute results, we defined acute technical, 30-day device and procedural success, as well as periprocedural complications, according to the Mitral Valve Academic Research Consortium (MVARC) criteria13. The primary study endpoint was all-cause mortality during a 2-year follow-up period in patients grouped according to extra-MV cardiac involvement. Secondary endpoints were cardiac death, first rehospitalisation for heart failure and a composite of overall death or rehospitalisation at 2-year follow-up.
STATISTICAL ANALYSIS
Distribution of continuous data was tested with the Shapiro-Wilk test. Normally distributed variables are expressed as mean±standard deviation, whereas non-normally distributed variables are presented as median and interquartile range. Categorical variables are reported as absolute values and corresponding percentages. Differences in continuous variables were tested using 1-way analysis of variance; categorical variables were compared with the Chi-square test. Paired comparison between baseline and follow-up variables was performed with the paired-sample Student’s t-test or Wilcoxon signed-rank test, as appropriate. Adverse events are reported as observed number of events and as Kaplan-Meier estimated rates. Event-free survival up to 2 years was evaluated according to the unadjusted Kaplan-Meier method, and survival among subgroups was compared using the log-rank test (Cox-Mantel test). Cox proportional hazards regression analysis was used to determine significant predictors of primary and secondary clinical endpoints. Variables with a univariate statistical significance of <0.1 were selected for inclusion in the multivariable model. Finally, multivariate analysis, using stepwise forward selection, was performed to analyse the association of baseline characteristics with study endpoints, expressed as hazard ratio (HR) with 95% confidence interval (CI) and p-values. All statistical tests were 2-sided, and p-values<0.05 were considered statistically significant. The statistical analyses were performed using SPSS software version 28.0.0 (IBM) and GraphPad Prism software version 8 (GraphPad).
Results
BASELINE CLINICAL AND ECHOCARDIOGRAPHIC CHARACTERISTICS
In the present analysis, a total of 654 patients with moderate-to-severe (3+) and severe (4+) PMR who underwent a TEER procedure were included (mean age 80±8 years; 53% male) (Figure 1). When patients were grouped according to extra-MV cardiac involvement, 9% (n=58) were in the NI group, 52% (n=343) in the LHI group and 39% (n=253) in the RHI group (Central illustration).
Patients with LHI were significantly younger when compared to the other groups; however, the prevalence of male gender was not different among the study groups. The RHI group exhibited higher surgical mortality risk, assessed by the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II, accompanied by lower median baseline haemoglobin levels and estimated glomerular filtration rates.
Baseline MR severity was comparable among groups. Consistent with a priori definitions, the largest LV dimensions were observed in patients with LHI. Accordingly, significant tricuspid regurgitation and more impaired right ventricle to pulmonary circulation coupling characterised the RHI group. Baseline clinical and echocardiographic characteristics of the entire study cohort and subgroups are reported in Table 1.
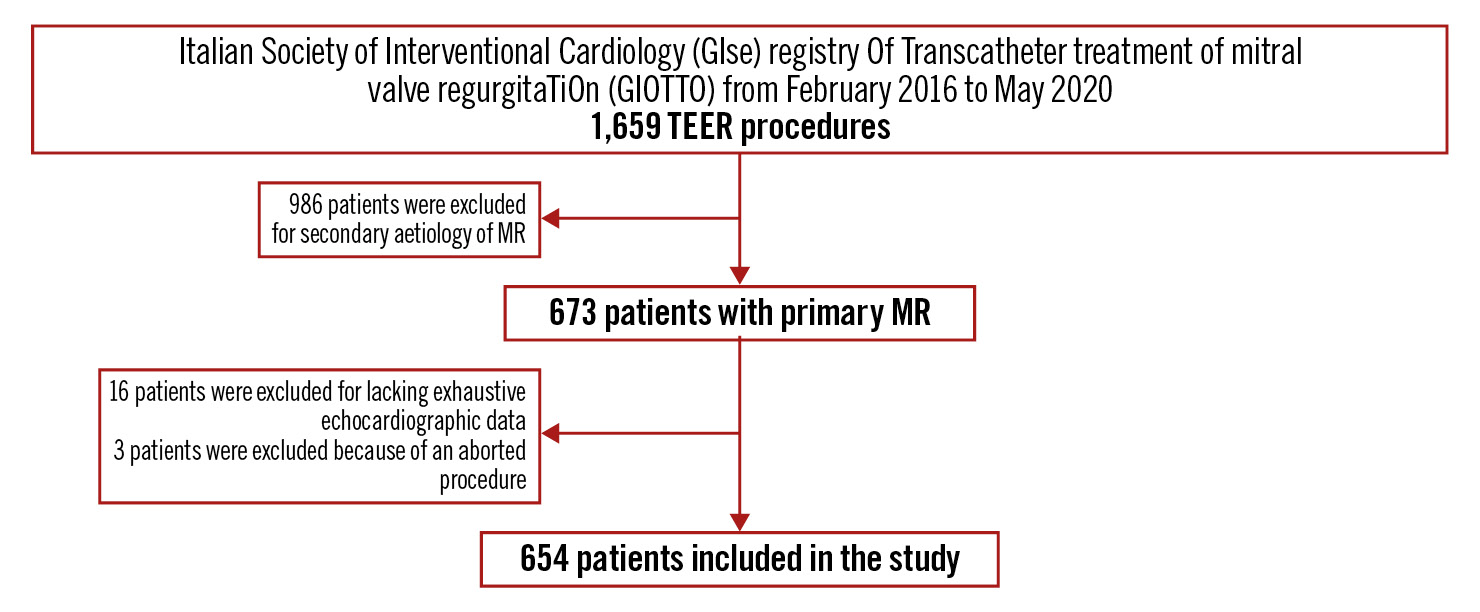
Figure 1. Study flowchart. MR: mitral regurgitation; TEER: transcatheter edge-to-edge repair
Table 1. Baseline clinical and echocardiographic characteristics of the entire study cohort and the three subgroups identified according to extra-mitral valve cardiac involvement.
| Entire study cohort (n=654) | NI (n=58) | LHI (n=343) | RHI (n=253) | p-value | |
|---|---|---|---|---|---|
| Demographic and clinical features | |||||
| Age, years | 80±8 | 81±7 | 78±8 | 81±7 | 0.001 |
| Male gender | 348 (53) | 29 (50) | 192 (56) | 127 (50) | 0.330 |
| BMI, kg/m2 | 25±4 | 24±4 | 25±4 | 25±4 | 0.298 |
| BSA, m2 | 1.76±0.20 | 1.75±0.21 | 1.78±0.21 | 1.76±0.19 | 0.410 |
| Previous or current smoker | 62 (17) | 4 (12) | 33 (19) | 25 (17) | 0.614 |
| EuroSCORE II, % | 3.9 [2.4; 5.7] | 3.1 [2.0; 4.7] | 3.6 [2.2; 5.4] | 4.5 [2.8; 6.1] | 0.002 |
| NYHA Class III-IV | 493 (75) | 30 (52) | 259 (76) | 204 (81) | <0.001 |
| Haemoglobin, g/dL | 12 [11; 14] | 13 [11; 14] | 13 [12; 14] | 12 [11; 13] | 0.012 |
| eGFR, mL/min/1.73 m2 | 44 [31; 58] | 46 [34; 65] | 46 [33; 60] | 40 [28; 52] | <0.001 |
| NT-proBNP, pg/mL | 494 [237; 1,702] | 360 [250; 916] | 421 [220; 1,855] | 568 [305; 2,544] | 0.356 |
| Comorbidities | |||||
| Hypertension | 478 (73) | 43 (74) | 252 (74) | 183 (72) | 0.936 |
| Diabetes mellitus | 119 (18) | 9 (16) | 55 (16) | 55 (22) | 0.175 |
| Dyslipidaemia | 165 (41) | 12 (33) | 91 (44) | 62 (39) | 0.323 |
| Coronary artery disease | 169 (26) | 8 (14) | 97 (28) | 64 (25) | 0.064 |
| History of MI | 118 (18) | 5 (9) | 65 (19) | 48 (19) | 0.148 |
| Previous CABG | 57 (9) | 3 (5) | 32 (9) | 22 (9) | 0.583 |
| Previous mitral valve repair/replacement | 14 (2) | 1 (2) | 9 (3) | 4 (2) | 0.667 |
| Previous TAVI | 19 (3) | 0 (0) | 7 (2) | 12 (5) | 0.059 |
| Atrial fibrillation | 337 (52) | 0 (0) | 188 (55) | 149 (59) | <0.001 |
| Previous hospitalisation for HF | 322 (49) | 23 (40) | 162 (47) | 137 (54) | 0.077 |
| CKD | 315 (48) | 20 (35) | 150 (44) | 145 (57) | <0.001 |
| COPD | 97 (15) | 9 (16) | 49 (14) | 39 (15) | 0.918 |
| PAD | 98 (29) | 7 (27) | 57 (33) | 34 (24) | 0.208 |
| Previous stroke | 39 (6) | 2 (3) | 22 (6) | 15 (6) | 0.677 |
| Devices | |||||
| ICD | 59 (9) | 0 (0) | 37 (11) | 22 (9) | 0.029 |
| CRT | 106 (16) | 2 (3) | 62 (18) | 42 (17) | 0.020 |
| Drugs | |||||
| ACEi/ARB/ARNI | 215 (33) | 29 (50) | 113 (33) | 73 (29) | 0.008 |
| Beta blockers | 475 (73) | 26 (45) | 260 (76) | 189 (75) | <0.001 |
| K+-sparing diuretics | 245 (38) | 13 (22) | 126 (37) | 106 (42) | 0.020 |
| Loop diuretics, mg | 50 [25; 100] | 25 [25; 50] | 50 [25; 75] | 50 [25; 100] | 0.058 |
| Anticoagulant therapy | 305 (47) | 0 (0) | 175 (51) | 130 (51) | <0.001 |
| Antiplatelet therapy | 297 (46) | 36 (62) | 153 (45) | 108 (43) | 0.027 |
| Mitral valve | |||||
| MR degree | 0.077 | ||||
| Moderate-to-severe | 113 (17) | 6 (10) | 72 (21) | 35 (14) | |
| Severe | 541 (83) | 52 (90) | 271 (79) | 218 (86) | |
| *VC, mm | 7 [5; 7] | 7 [3; 8] | 7 [5; 8] | 7 [6; 7] | 0.496 |
| †EROA, cm2 | 0.4 [0.3; 0.5] | 0.4 [0.3; 0.4] | 0.4 [0.3; 0.5] | 0.5 [0.4; 0.5] | 0.374 |
| MV area | 4.8±1.3 | 4.5±1.0 | 4.9±1.3 | 4.9±1.3 | 0.142 |
| Left ventricular dimensions and function | |||||
| LVEDD, mm | 55±9 | 51±6 | 56±9 | 55±9 | 0.001 |
| LVESD, mm | 37±11 | 29±6 | 39±11 | 37±11 | <0.001 |
| LVEDV, mL | 126±51 | 104±28 | 131±52 | 126±52 | 0.003 |
| LVEDVi, mL/m2 | 71±27 | 59±13 | 73±28 | 71±29 | 0.003 |
| LVESV, mL | 60±36 | 34±11 | 65±37 | 60±36 | <0.001 |
| LVESVi, mL/m2 | 34±19 | 19±5 | 36±20 | 34±19 | <0.001 |
| LVEF, % | 53±12 | 68±4 | 50±12 | 54±12 | <0.001 |
| E/e’ | 13 [10; 18] | 11 [10; 13] | 13 [10; 18] | 13 [10; 19] | 0.336 |
| Left atrial dimensions | |||||
| LAD, mm | 50±11 | 43±6 | 50±11 | 51±12 | <0.001 |
| Right ventricle | |||||
| TR degree | <0.001 | ||||
| None | 22 (3) | 4 (7) | 16 (4) | 2 (1) | |
| Mild | 264 (40) | 39 (67) | 184 (54) | 41 (16) | |
| Moderate | 266 (41) | 15 (26) | 143 (42) | 108 (43) | |
| Severe | 102 (16) | 0 (0) | 0 (0) | 102 (40) | |
| TAPSE, mm | 20±7 | 24±4 | 21±9 | 18±5 | 0.002 |
| sPAP, mmHg | 48±15 | 38±8 | 39±8 | 61±13 | <0.001 |
| TAPSE/sPAP, mm/mmHg | 0.47±0.22 | 0.67±0.20 | 0.57±0.22 | 0.33±0.12 | <0.001 |
| Data are presented as n (%) or mean±SD or median [IQR]. *VC values are available for ~30% of the entire study cohort. †EROA values are available for ~20% of the entire study cohort. ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin 1 receptor blocker; AF: atrial fibrillation; ARNI: angiotensin receptor-neprilysin inhibitor; BMI: body mass index; BSA: body surface area; CABG: coronary artery bypass graft; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronisation therapy; E/e’: early mitral inflow velocity to mitral annular early diastolic velocity ratio at tissue Doppler imaging; eGFR: estimated glomerular filtration rate; EROA: effective regurgitant orifice area; EuroSCORE: European System for Cardiac Operative Risk Evaluation; HF: heart failure; i: index; ICD: implantable cardioverter-defibrillator; IQR: interquartile range; K+: potassium; LAD: left atrial diameter; LHI: left heart involvement; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume; MI: myocardial infarction; MV: mitral valve; MR: mitral regurgitation; MV: mitral valve; NI: no extra-mitral valve involvement; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; NYHA: New York Heart Association; PAD: peripheral artery disease; RHI: right heart involvement; RV: right ventricle; SD: standard deviation; S’ TDI: systolic wave velocity at tissue Doppler imaging; sPAP: systolic pulmonary arterial pressure; TAPSE: tricuspid annular plane systolic excursion; TAVI: transcatheter aortic valve implantation; TR: tricuspid regurgitation; VC: vena contracta | |||||
PROCEDURAL OUTCOMES
MVARC technical success was achieved in 638 patients (98%), with no significant differences between the study groups, and overall sustained 30-day device and procedural success rates (88% and 86%, respectively). Of note, the RHI group showed the lowest percentage of device and procedural success (81% and 79%, respectively), with significant differences when compared to the other study groups. Among the 16 patients with unsuccessful MitraClip implantation, 5 (1%) had in-hospital single leaflet device attachment, with no significant differences among the groups. The number of implanted devices and residual MR ≥3+ at the end of the TEER procedure did not differ between groups, nor the rate of acute heart failure, acute kidney injury, and periprocedural bleeding. In-hospital mortality was 2% (13 patients) and was significantly more frequent in the RHI group, whereas no statistically significant difference was observed for in-hospital cardiac death. All procedural data are presented in Table 2.
Table 2. Procedural and 30-day outcomes of the entire study cohort and the three subgroups identified according to extra-mitral valve cardiac involvement.
| Procedural outcomes | Entire study cohort (n=654) | NI (n=58) | LHI (n=343) | RHI (n=253) | p-value |
|---|---|---|---|---|---|
| MVARC technical success | 638 (98) | 57 (98) | 337 (98) | 244 (96) | 0.344 |
| Device implanted | 0.011 | ||||
| MitraClip | 155 (24) | 8 (14) | 90 (26) | 57 (23) | |
| MitraClip NT | 220 (33) | 15 (26) | 111 (33) | 94 (37) | |
| MitraClip NTr | 89 (14) | 6 (10) | 45 (13) | 38 (15) | |
| MitraClip XTr | 190 (29) | 29 (50) | 97 (28) | 64 (25) | |
| Implanted clips | 0.649 | ||||
| 1 | 270 (41) | 27 (47) | 146 (43) | 97 (38) | |
| 2 | 323 (49) | 29 (50) | 165 (48) | 129 (51) | |
| ≥3 | 61 (10) | 2 (3) | 32 (9) | 27 (11) | |
| MR ≥3+ | 29 (4) | 1 (2) | 14 (4) | 14 (6) | 0.401 |
| Acute HF | 13 (2) | 1 (2) | 6 (2) | 6 (2) | 0.856 |
| AKI | 14 (2) | 1 (2) | 9 (3) | 4 (2) | 0.667 |
| Minor/major/disabling bleeding | 44 (7) | 4 (7) | 26 (8) | 14 (6) | 0.614 |
| Partial clip detachment | 5 (1) | 0 (0) | 1 (0.3) | 4 (2) | 0.159 |
| †In-hospital death | 13 (2) | 0 (0) | 2 (1) | 11 (4) | 0.004 |
| †In-hospital cardiac death | 8 (1) | 0 (0) | 2 (1) | 6 (3) | 0.110 |
| 30-day outcomes | |||||
| MVARC device success | 574 (88) | 54 (93) | 314 (92) | 206 (81) | <0.001 |
| MVARC procedural success | 559 (86) | 54 (93) | 306 (89) | 199 (79) | <0.001 |
| Data are presented as n (%). †p-values are generated by Cox-Mantel analysis. AKI: acute kidney injury; HF: heart failure; LHI: left heart involvement; MR: mitral regurgitation; MVARC: Mitral Valve Academic Research Consortium; NI: no extra-mitral valve involvement; RHI: right heart involvement | |||||
MIDTERM FOLLOW-UP DATA
With 113 (17%) patients lost to follow-up, a total of 541 were followed for a median of 22 (12-24) months. One-year survival differences indicate worse outcomes with increasing extra-MV cardiac involvement (unadjusted HR per 1-group increase from NI to LHI and from LHI to RHI: 1.820, 95% CI: 1.229-2.695; p=0.003). According to 2-year Kaplan Meier analysis, the rate of all-cause mortality was significantly lower in the NI group (7%), compared to the LHI and RHI groups (both 27%) (Central illustration). Likewise, higher composite endpoint rates were observed in the cardiac involvement groups (p=0.047) (Figure 2A, Supplementary Table 1). In addition, the midterm cardiac death rate tended to be higher in the RHI group, whereas heart failure rehospitalisation occurred more frequently in patients with LHI, although, this did not reach statistical significance (Figure 2B, Figure 2C, respectively). Primary and secondary clinical endpoints at 1- and 2-year follow-up are shown in Table 3. At univariate Cox regression analysis, the subgroup of extra-MV cardiac involvement, together with age, New York Heart Association Functional Class ≥III, diabetes mellitus, haemoglobin concentration and technical success were able to predict the primary endpoint. On multivariable analysis, the subgroup of extra-MV cardiac involvement, haemoglobin level and technical success were found to be independent predictors of all-cause mortality (Table 4).
A significant improvement in New York Heart Association Functional Class and MR grade was observed at 2-year follow-up (Supplementary Figure 1, Supplementary Figure 2).
One- and 2-year changes in the main echocardiographic features are shown in Supplementary Table 2 and Supplementary Table 3, respectively.

Figure 2. Unadjusted Kaplan-Meier survival estimates for the occurrence of the secondary endpoints. Plots of survival free from the composite endpoint of all-cause death or rehospitalisation for heart failure (HF) (A), cardiac death (B) and HF rehospitalisation (C), in patients stratified according to extra-mitral valve cardiac involvement. P-values are bold only when statistically significant (<0.05). LHI: left heart involvement; NI: no extra-mitral valve involvement; RHI: right heart involvement; TEER: transcatheter edge-to-edge repair
Table 3.
Primary and secondary clinical endpoints in the entire study cohort and three subgroups identified according to extra-mitral valve cardiac involvement at 1- and 2-year follow-up.
| Entire study cohort (n=654) | NI (n=58) | LHI (n=343) | RHI (n=253) | p-value | |
|---|---|---|---|---|---|
| 1-year follow-up | |||||
| All-cause death | 78 (15) | 1 (3) | 36 (14) | 41 (20) | 0.010 |
| All-cause death and/or rehospitalisation for HF | 97 (18) | 2 (5) | 48 (18) | 47 (22) | 0.026 |
| Cardiac death | 35 (7) | 0 (0) | 14 (6) | 21 (11) | 0.017 |
| Rehospitalisation for HF | 33 (7) | 1 (3) | 18 (8) | 14 (8) | 0.516 |
| 2-year follow-up | |||||
| All-cause death | 108 (25) | 2 (7) | 57 (27) | 49 (27) | 0.041 |
| All-cause death and/or rehospitalisation for HF | 132 (31) | 3 (10) | 73 (34) | 56 (30) | 0.047 |
| Cardiac death | 46 (11) | 0 (0) | 23 (11) | 23 (13) | 0.071 |
| Rehospitalisation for HF | 39 (11) | 1 (3) | 23 (13) | 15 (9) | 0.396 |
Table 4. Primary endpoint-related univariate and multivariate Cox regression analysis in the entire study cohort.
| Univariate analysis* | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age, per 1-year increase | 1.338 | 0.919-1.948 | 0.092 | |||
| NYHA Class III/IV | 1.963 | 1.155-3.337 | 0.013 | |||
| Diabetes mellitus | 1.684 | 1.106-2.565 | 0.015 | |||
| Haemoglobin, per 1-g/dL increase | 0.791 | 0.700-0.895 | <0.001 | 0.801 | 0.703-0.912 | 0.001 |
| Technical success | 0.163 | 0.082-0.324 | <0.001 | 0.164 | 0.062-0.435 | <0.001 |
| NI | reference | reference | ||||
| LHI | 4.315 | 1.053-17.675 | 0.344 | 4.726 | 1.116-20.010 | 0.035 |
| RHI | 5.184 | 1.261-21.308 | 0.022 | 4.245 | 1.010-17.845 | 0.048 |
| Data are presented as hazard ratio (HR) with 95% confidence interval (CI) and p-values. *Only statistically significant covariates are reported. LHI: left heart involvement; NI: no extra-mitral valve involvement; NYHA: New York Heart Association; RHI: right heart involvement | ||||||
Discussion
In this real-world multicentre experience of symptomatic patients with moderate-to-severe and severe PMR treated with MitraClip, we found the following:
a) preoperative extra-MV cardiac involvement is a frequent occurrence, with fewer than 10% of patients presenting with no extra-MV involvement;
b) even though study group patients represent distinct clinical and echocardiographic entities, the TEER procedure with the MitraClip device was performed safely and was demonstrated to be effective in terms of acute technical success in the entire study cohort. However, the RHI group showed lower rates of MVARC device and procedural success;
c) all-cause mortality considered singularly, as well as a composite endpoint of overall death and rehospitalisation for heart failure, occurred more frequently in the extra-MV cardiac involvement groups; however, no differences were observed for cardiac death or heart failure hospitalisation alone;
d) being in ≥1 group of extra-MV cardiac involvement represents in itself a negative predictor of midterm outcomes.
MECHANISM AND PREVALENCE OF EXTRA-MV CARDIAC INVOLVEMENT IN PMR
MR induces volume and pressure overload in the left chambers and imposes an increased pulsatile loading on the pulmonary circulation14. Indeed, the early haemodynamic derangement induced by chronic MR is an increase in stroke volume, resulting in eccentric myocardial hypertrophy and progressive LV dilation as compensatory mechanisms to normalise wall stress. However, LV contractility gradually declines. The left atrium also progressively dilates with a parallel increase in its compliance to counterbalance the increase in pressure. As the left atrium buffers pressure and flow oscillations during the cardiac cycle, when its function is impaired, a further haemodynamic stress on the pulmonary circulation takes place and favours an increase in lung capillary hydrostatic pressure. In this framework, the occurrence of atrial fibrillation is crucial in further promoting impaired LV filling, increasing pulmonary venous pressure and contributing to atrial remodelling. Right atrial enlargement might elicit tricuspid annulus dilation, leading to leaflet malcoaptation and, ultimately, tricuspid regurgitation (atrial-predominant phenotype). Long-standing elevated pulmonary pressure in turn often induces right ventricular dilation and the development of secondary tricuspid regurgitation, which ultimately results in right ventricular dysfunction (ventricular-predominant phenotype)15. The aforementioned is generally a timeline progression, as suggested by the 93% overlap of LHI criteria in the RHI group. Nevertheless, this pathophysiological cascade might also occur in different ways, suggesting the influence of patient-related factors and comorbidities (e.g., underlying pulmonary disease), leading to distinct haemodynamic adaptations in response to severe MR.
Overall, the prevalence of different phenotypes of extra-MV cardiac involvement in candidates for TEER for significant PMR has never been comprehensively investigated. According to the results of our study, depicting a real-world contemporary cohort, 80% of patients presented with LV dilation and/or dysfunction: 58% with left atrium involvement, 38% with increased pulmonary artery pressure and/or significant tricuspid regurgitation, and 9% with impaired right ventricle to pulmonary circulation coupling. In a recent registry of patients undergoing TEER with PMR as the most prevalent aetiology, LV dysfunction (defined as LV ejection fraction <50%) and LV dilation (defined as LV end-systolic volume ≥40 mm) have been reported in 35% and 32% of patients, respectively16. In our study, the prevalence of atrial fibrillation is consistent with previous studies, in which atrial fibrillation burden was found to be as high as 50-67%1718. By contrast, the observed prevalence of pulmonary circulation and right ventricular involvement is lower than reported by Shamekhi et al19, likely because of the different echocardiographic parameters used for defining right heart remodelling.
ACUTE AND 30-DAY PROCEDURAL RESULTS ACCORDING TO EXTRA-MV CARDIAC INVOLVEMENT
Despite distinct baseline clinical and echocardiographic features according to the study groups, TEER with the MitraClip device was performed safely in all patient groups and was demonstrated to be effective in terms of acute technical success, showing that extra-MV cardiac involvement per se does not affect acute results. Similarly, no significant differences were observed across the spectrum of extra-MV cardiac involvement with respect to acute kidney injury, bleeding complications or acute heart failure rates. However, as regards 30-day procedural outcomes, RHI patients showed lower rates of MVARC device and procedural success, as well as more frequent in-hospital death. These findings likely suggest that this phenotype deserves more clinical attention, especially in case of suboptimal postprocedural results720.
MIDTERM PROGNOSTIC IMPACT OF CARDIAC INVOLVEMENT IN PRIMARY MR
The frailty of patients with LV dilatation and dysfunction, new-onset atrial fibrillation, or pulmonary hypertension, even in the absence of symptoms, has been underlined by the indications for MV surgery in current guidelines4. However, these red flags for procedural timing have been neglected in those patients who are unsuitable for cardiac surgery. In line with this, we sought to characterise extra-MV cardiac involvement phenotypes by combining different parameters exploring both left and right heart anatomy and function and assessing their prognostic value. Accordingly, in the present study, an intuitive and practical classification system was applied including factors mirroring the extent of extra-MV cardiac involvement, which were tested in a real-world setting. The examined parameters were selected from those associated with adverse outcomes in previous studies2122232425.
To the best of our knowledge, this study is the first midterm analysis showing the association between heart chamber involvement and worse midterm outcome in terms of all-cause mortality in patients with PMR treated with TEER. Interestingly, while RHI exhibited the lowest survival rate at 1-year follow-up, Kaplan Meier analysis did not reveal significant differences with LHI after 2 years following the procedure. This may be partly explained by a decreasing sample size and lower number of events. Additionally, a certain degree of group crossover should be considered, since LHI patients may develop RHI as the duration of follow-up increases. Nevertheless, we cannot assume MitraClip treatment to be futile in these patients, since symptomatic improvement was observed at 2-year follow up regardless of the burden of extra-MV involvement.
The rationale for early treatment would be to prevent pathological changes from occurring, thus preserving normal ventricular and atrial chamber dimensions and function, sinus rhythm, and better long-term valve function. Consistently, early MV surgery is associated with better long-term outcomes in terms of survival and new-onset heart failure, compared with initial medical management26. Our results corroborate the hypothesis that this could also be the case in the transcatheter framework. Nevertheless, whether early TEER treatment might prevent progression to prognostically less favourable cardiac remodelling phenotypes remains to be determined in prospective trials.
Recent studies have demonstrated the utility of characterising the extent of cardiac involvement among patients presenting with severe aortic stenosis27, moderate and severe secondary MR2829, or severe PMR undergoing surgery11. In line with this, our findings suggest that more emphasis should be given to a systematic and comprehensive evaluation of anatomical and functional cardiac remodelling associated with PMR as a meaningful element impacting on midterm prognosis after transcatheter repair. The identification of extra-MV cardiac involvement can be easily performed and, therefore, could be taken into consideration in patient selection, to improve risk stratification and to potentially guide the timing of an intervention.
Limitations
The present study has some limitations that should be acknowledged. The most relevant is related to its non-randomised, observational design. Patient selection and ascertainment bias may have influenced event rates. Although we performed a multivariable Cox regression model with a large number of covariates, the influence of unmeasured confounders cannot be excluded. Moreover, the evaluation of MR was based mainly on qualitative parameters. However, it is worth mentioning that in MV prolapse or leaflet flail, regurgitant jets are often very eccentric, and poor alignment does not allow for adequate quantification of MR severity30. In addition, an independent core lab to adjudicate echocardiographic data was not available, and a uniform protocol for postprocedural MR assessment was not clearly established. Lastly, midterm follow-up data were obtained with clinical visits (40%) and telephone calls (60%), and, therefore, outpatient assessments including New York Heart Association Functional Class and echocardiography at follow-up were incomplete and did not allow evaluation of the evolution of cardiac remodelling. In view of this, our results should be considered as hypothesis-generating, stimulating further investigations into an optimal treatment strategy (timing and selection) in patients suffering from PMR.
Conclusions
The classification of patients with PMR undergoing TEER into groups according to extra-MV cardiac involvement provides prognostic value in terms of postinterventional outcome. Grading cardiac remodelling may help refine risk stratification and timing of the procedure, since being in ≥1 group of extra-MV cardiac involvement represents in itself a negative predictor of midterm outcome.
Impact on daily practice
Adequate risk stratification and timely indication for TEER in patients with severe primary MR remain a clinical challenge. This new classification system can be easily performed and suggests that more emphasis should be given to a systematic and comprehensive evaluation of anatomical and functional cardiac remodelling associated with PMR. Extra-MV cardiac involvement phenotyping might be taken into consideration in patient selection to improve the management and potentially guide the timing of an intervention. Larger prospective studies will be necessary to confirm whether early transcatheter treatment of PMR might prevent disease progression to prognostically less favourable cardiac remodelling phenotypes.
Funding
The GIOTTO Registry is sponsored by the Italian Society of Interventional Cardiology (GISE), which received grant support for the study from Abbott.
Conflict of interest statement
M. Adamo, C. Grasso, P. Denti, A. Giordano, A.L. Bartorelli, M. Montorfano, A.S. Petronio, C. Tamburino and F. Bedogni received consultation and/or speaker fees from Abbott, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

