Abstract
Background: An independent panel of experts reviewed all investigator-reported cases of mitral valve leaflet adverse events (LAE) after MitraClip NTR/XTR in the EXPAND study.
Aims: We aimed to report the findings of the expert panel and standardise definitions for LAE.
Methods: Standard definitions for different types of LAE were formulated and events adjudicated after detailed review by the expert panel.
Results: Enrolling centres reported LAE in 35 cases, 11 leaflet injuries (9 tears, 2 perforations) and 24 single leaflet device attachment (SLDA). The panel confirmed LAE in 20 cases (2.0% incidence), 18 patients had SLDA and 4 had leaflet injury (2 cases had both SLDA and injury). Leaflet injury occurred during device implant and resulted in surgical valve replacement or death. SLDA-alone events were identified during implant (n=2), pre-discharge (n=7) or at 30 days of follow-up (n=7) and were resolved (≤2+ residual MR) with additional clips in 75% of cases.
Conclusions: Mitral valve repair with MitraClip NTR/XTR is safe. The rate of LAE is lower than previously reported using older-generation devices. The proposed definitions and findings will help to differentiate leaflet injury from inadequate leaflet insertion and SLDA and provide guidance for consistent diagnosis of LAE post MitraClip implantation.
Introduction
Transcatheter mitral valve repair (TMVr) through edge-to-edge leaflet approximation with the MitraClip™ device (Abbott Vascular, Santa Clara, CA, USA) is an approved therapy that is widely used and has been supported by large randomised clinical trials demonstrating its safety and efficacy in patients with primary and secondary mitral regurgitation (PMR, SMR)1,2,3,4. Despite its track record of safety, a small, albeit important number of device-related leaflet adverse events (LAE) have been reported5. In real-world clinical practice, however, there are limited safety data available on LAE6,7. Importantly, there has been a lack of unified definitions to characterise LAE in a standardised manner.
The EXPAND study is prospective and reflects real-world contemporary practice with the MitraClip NTR and XTR systems globally, including PMR and SMR patients. During the initial analysis, LAE were reported by the enrolling centres in 3.4% of the patients. While various factors could be associated with LAE, an in-depth assessment to identify the root cause of such events was difficult due to the lack of standardisation in LAE-specific image acquisition and variations in terminology for the definition of various forms of LAE. In concert, these circumstances have resulted in reporting inconsistencies across the study sites, which in turn has complicated the proper understanding of the incidence and mechanisms leading to LAE. The EXPAND study collected standardised echocardiographic and clinical data from index procedure up to one year of follow-up, providing a unique opportunity for an independent committee to adjudicate the presence and nature of LAE associated with the MitraClip procedure.
Accordingly, our aim was to report the incidence of LAE in this global post-market study, to provide details on the different types of LAE encountered and to propose practical standardised definitions for LAE. These definitions will be critical to facilitate proper recognition and homogeneous reporting of LAE in future clinical trials and in clinical practice.
Methods
EXPAND STUDY DESIGN
EXPAND is a prospective, multicentre, single-arm, post-market, real-world observational study conducted at 57 centres in Europe, the Middle East and the USA. Enrolling centres were required to have experience with previous generations of the MitraClip device and had to have performed at least three cases with the NTR/XTR systems prior to enrolment in the study. A minimum of 1,000 consecutive subjects with PMR or SMR undergoing TMVr with third-generation MitraClip NTR and XTR systems were authorised for the EXPAND study. In accordance with clinical practice guidelines, patients were enrolled if they had symptomatic 3+ or 4+ mitral regurgitation (MR) and were deemed candidates for MitraClip implant by the Heart Teams at the enrolling institutions8,9,10. Follow-up was conducted per standard of care at 30 days, 6 and 12 months and at times of adverse events. All echocardiograms (transoesophageal and transthoracic) were analysed by two independent echocardiography core laboratories (ECL), the first ECL for MR aetiology, MR severity and valve complexity, and the second ECL for detailed mitral valve anatomic measurements. Major adverse events were adjudicated by an independent clinical events committee. Device-related LAE, including single leaflet device attachment (SLDA), leaflet injury, device embolisation or mitral stenosis, were initially reported by the enrolling centres in the absence of standard definitions for LAE.
MULTIDISCIPLINARY EXPERT PANEL
A panel of independent experts including interventional echocardiographers, interventional cardiologists and cardiovascular surgeons was convened to review all cases of LAE reported by the enrolling centres between the time of implant and 12 months of follow-up. This team of multidisciplinary experts was built to provide a wide range of perspectives to complement each other’s expertise and provide a comprehensive analysis of each case. The study sponsor (Abbott, Santa Clara, CA, USA) assisted in collecting the data requested by the panel but did not participate in any discussion or outcome adjudication. The evaluation included review of all echocardiograms related to the LAE, TMVr procedure and follow-ups, as well as all clinical data, procedural reports and surgical or autopsy reports when available. The panel met through video conferences and in person for group discussion of every case. The goal of this panel was to adjudicate the LAE and to formulate standard echocardiographic definitions for different types of LAE based on the observations from the EXPAND study and the panel member’s clinical expertise (Central illustration). The panel chair (F.M. Asch) and members had full access to all data provided by the clinical centres and take full responsibility for data completion and integrity.
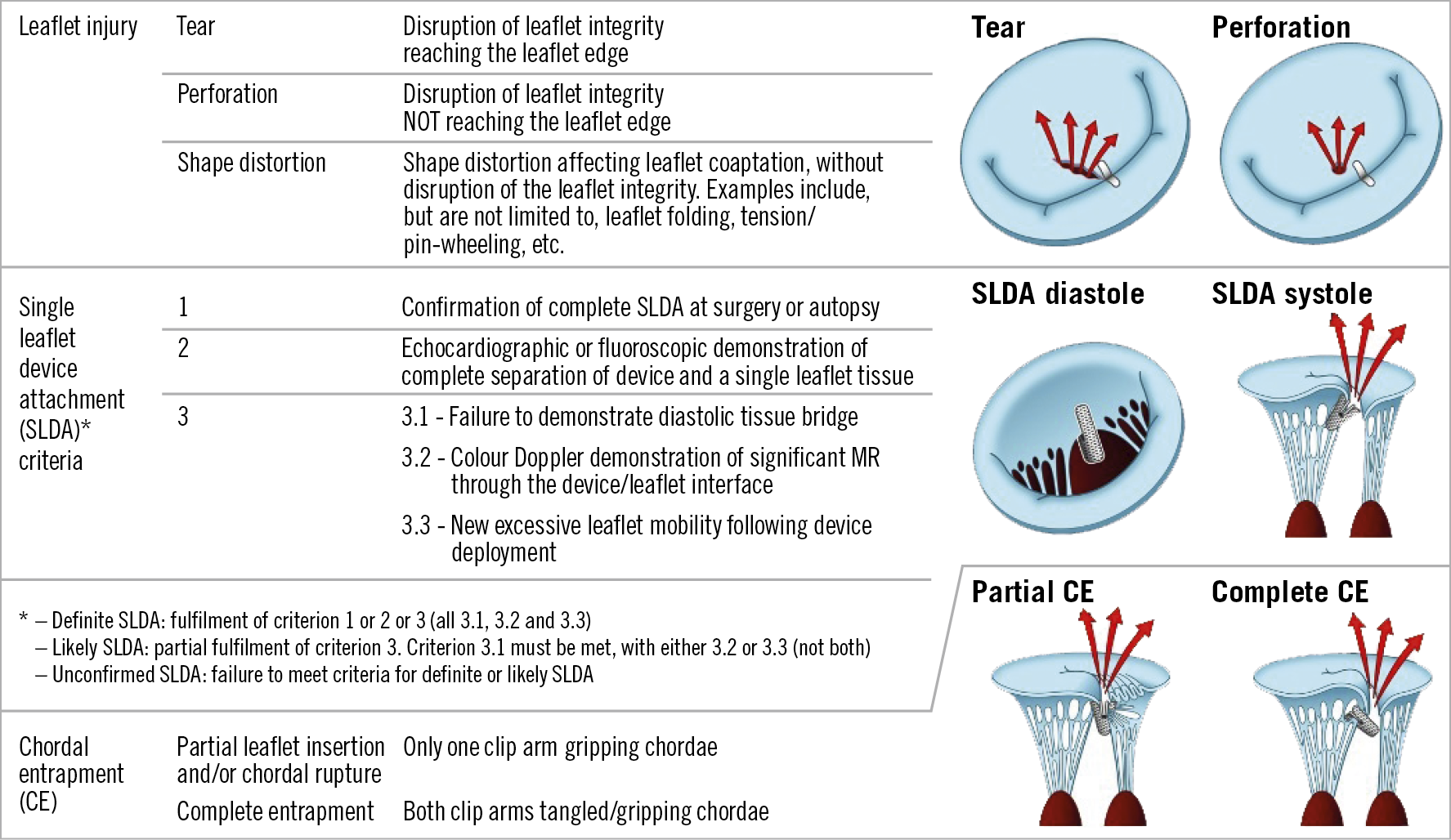
Central illustration. Classification and definitions of MitraClip-related leaflet adverse events.
CASE REVIEWS
EXPAND study sites reported 11 cases of leaflet injury and 24 cases of isolated SLDA in subjects treated with MitraClip NTR and XTR systems. Upon detailed review of each case, LAE were adjudicated by the panel. The panel reviewed the echocardiographic images and all available clinical information and reached an adjudication decision by consensus, which required agreement between at least 2/3 of the panel members. The adjudication results indicate whether the reported event was deemed as no LAE, a leaflet injury (perforation or tear), chordal entrapment and/or SLDA based on the images and clinical evidence provided by the enrolling centres. Each leaflet injury case was also adjudicated for residual MR, presence of mitral valve anatomic complexity including small mitral valve orifice, primary jet outside of the A2-P2 coaptation zone, wide jet, more than one significant MR jet, minimal leaflet tissue for attachment, cleft or calcification in the grasping zone.
DEFINITIONS OF LAE
The specific echocardiographic and clinical characteristics of each type of LAE were formulated to ensure consistency in definitions and diagnosis of MitraClip-related LAE. The types of LAE included leaflet perforation, leaflet tear, leaflet shape distortion, partial leaflet gripping, chordal entrapment and SLDA and are described in the Central illustration. SLDA was defined by three criteria based on surgical or anatomic reports (criterion 1), an echocardiographic or fluoroscopic demonstration of complete separation between device and leaflet tissue (criterion 2) or combination of imaging-specific anatomic (criterion 3.1 – failure to demonstrate diastolic tissue bridge, criterion 3.3 – new excessive leaflet mobility following device deployment), and MR (criterion 3.2 – new-onset significant MR) descriptors. Definite SLDA definition required fulfilment of criteria 1 or 2 or 3 (all descriptors under criterion 3). Likely SLDA required partial fulfilment of criterion 3 (3.1 must be met with either 3.2 or 3.3). The agreed definitions of types of LAE are described in the Central illustration.
Results
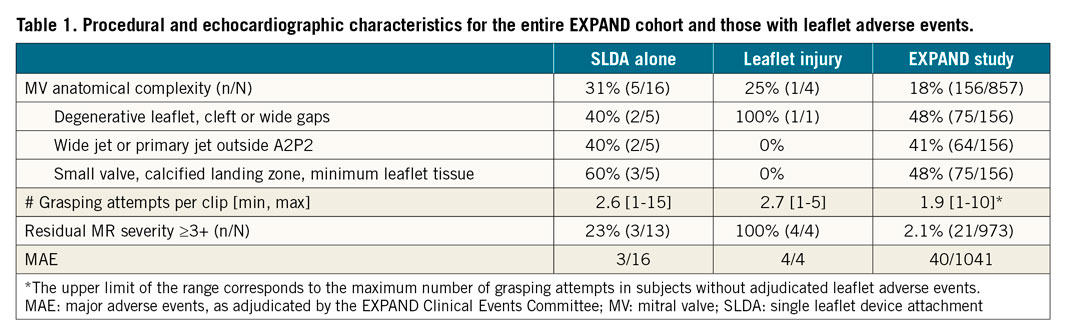
Between April 2018 and June 2019, 1,041 patients were enrolled in EXPAND. Patients were 77.3 years old (±9.7) and 54.9% were male. There were 909 subjects with baseline echocardiograms of acceptable quality; however, MR aetiology could not be determined by the echocardiography core lab in 74 of them. The aetiology of MR was considered to be PMR in 382 cases (46%), SMR in 413 (49%) and mixed in 40 (5%). Mitral valve complex anatomy was identified in 156 cases (18%), including complex degenerative leaflets with a wide gap (n=75), jet outside A2/P2 (n=17), small valves (n=7), calcified landing zone (n=52) and/or minimum leaflet tissue (n=16). Fifty-four patients (5.2%) had prior mitral valve procedures (surgical or transcatheter repair). The number of clips implanted per patient was 1.5±0.6 (416 received only NTR, 463 only XTR, and 151 received at least one of each). The average number of grasps attempted per clip implanted was 1.9 overall (range 1 to 15), 2.0 for cases receiving only NTR clips (range 1-10), 1.7 for those receiving only XTR (range 1-15), and 1.8 for those receiving at least one XTR (XTR-only or XTR and NTR, range 1-15). Procedural and echocardiographic characteristics for the entire EXPAND cohort and those with LAE are presented in Table 1.
SITE-REPORTED LAE
The enrolling centres reported LAE in 35 cases, of which 11 were reported as leaflet injury (9 tear, 2 perforation) and 24 as SLDA alone (without leaflet injury). No cases of isolated leaflet shape distortion or chordal entrapment were reported by the sites or identified by the adjudication committee.
INDEPENDENT COMMITTEE ADJUDICATION OF LAE
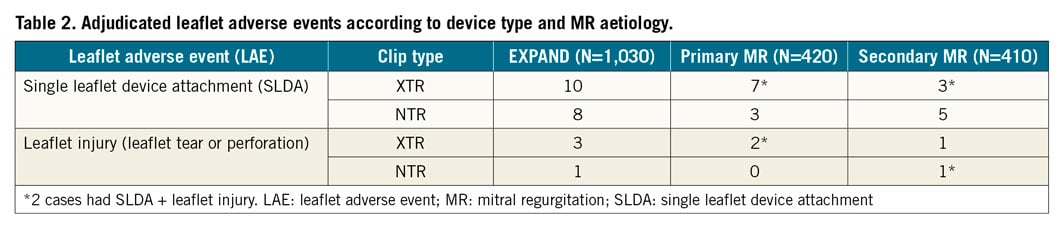
The adjudication committee confirmed LAE in 20 cases (2.0% incidence in the EXPAND study), 11 in patients with PMR and 9 in SMR (2.9% vs 2.4%, p=0.82) (Table 2). Among the patients with confirmed LAE who completed the study (n=14, 6 withdrew before the 12-month follow-up), 10 (71%) survived at least 12 months.
LEAFLET INJURY
Leaflet injury was confirmed in 4 cases (0.4% incidence), including 2 that had coexisting leaflet injury and SLDA (cases 1 and 2 below). The cases that were not confirmed as leaflet injury (n=7) presented with unresolved residual MR despite multiple grasping attempts. Disruption of leaflet integrity (tears or perforations) was not present in these cases and no obvious leaflet folding or tension could be identified. Three of the confirmed injuries were related to XTR devices and 1 to NTR. All cases were identified during the implant procedure. Only 1 of these cases had features of mitral valve anatomical complexity (a cleft) and all resulted in 3+ or 4+ MR. All events resulted in surgical mitral valve replacement and/or death. Detailed images of the 4 leaflet injury examples are shown in Figure 1-Figure 4.
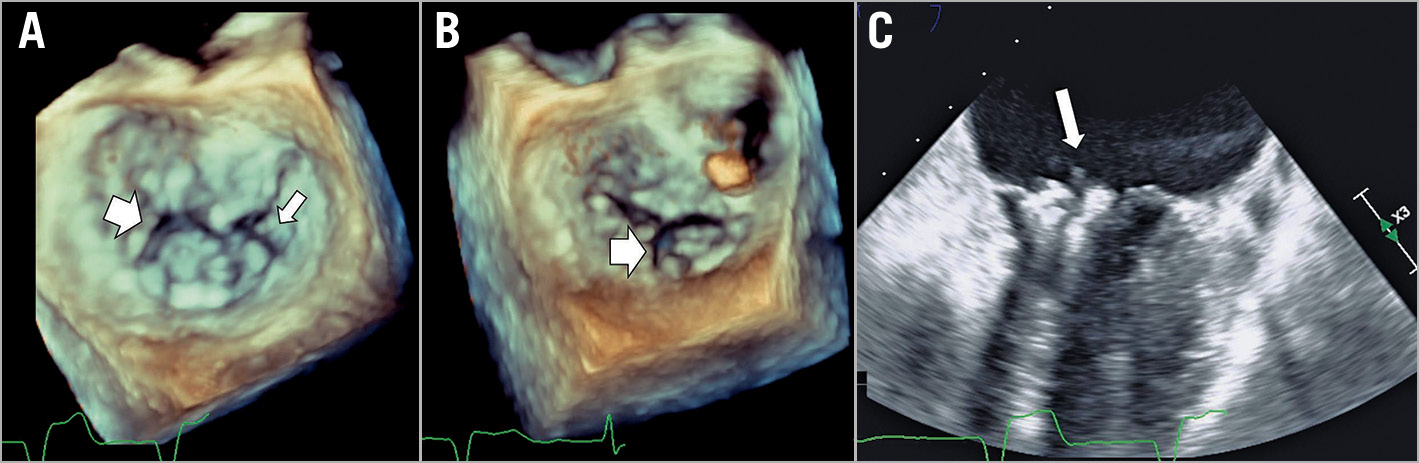
Figure 1. Example 1 with clefts, leaflet tear and SLDA. The baseline MV anatomy (A) showed primary MR with a lateral cleft (large arrow) and a medial small cleft (small arrow). The first clip (XTR, 3 grasping attempts) created tension in P2, increasing the opening of the lateral cleft (B). After a second, medial clip (XTR, 4 grasping attempts), the edge of the medial cleft got ripped, creating a tear of the posterior MVL (arrow, C) and resulted in SLDA (detached from the posterior MVL). The resulting severe MR was not resolved with a third clip (NTR, 1 grasp). Due to 4+ MR, the patient underwent successful emergency surgical valve replacement, and the surgeon reported a tear with a small perforation on the medial aspect of P2. The patient was discharged to a rehabilitation facility on postoperative day 26 and withdrew from the study at day 30 based on the physician’s decision.
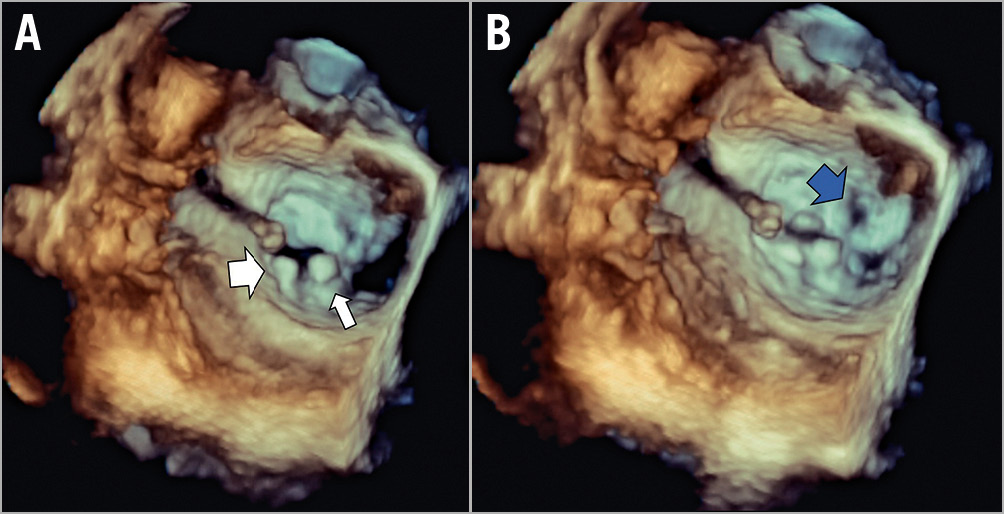
Figure 2. Example 2 was a patient with 4+ SMR and tethering of P2, complicated with SLDA and anterior leaflet perforation. A) The central clip (XTR, 2 grasping attempts) is well placed in A2/P2 position with proper diastolic bridging (small arrow), while the lateral clip (NTR, 3 attempts) is attached only to the posterior leaflet (detached from the anterior leaflet, loss of leaflet bridging in diastole, large arrow). B) In systole the perforation in the anterior leaflet (blue arrow) is shown. A third clip (NTR, 1 attempt) did not resolve the MR and the patient underwent successful emergency surgical valve replacement, was discharged home and was eventually withdrawn from the study at day 120 by the physician.
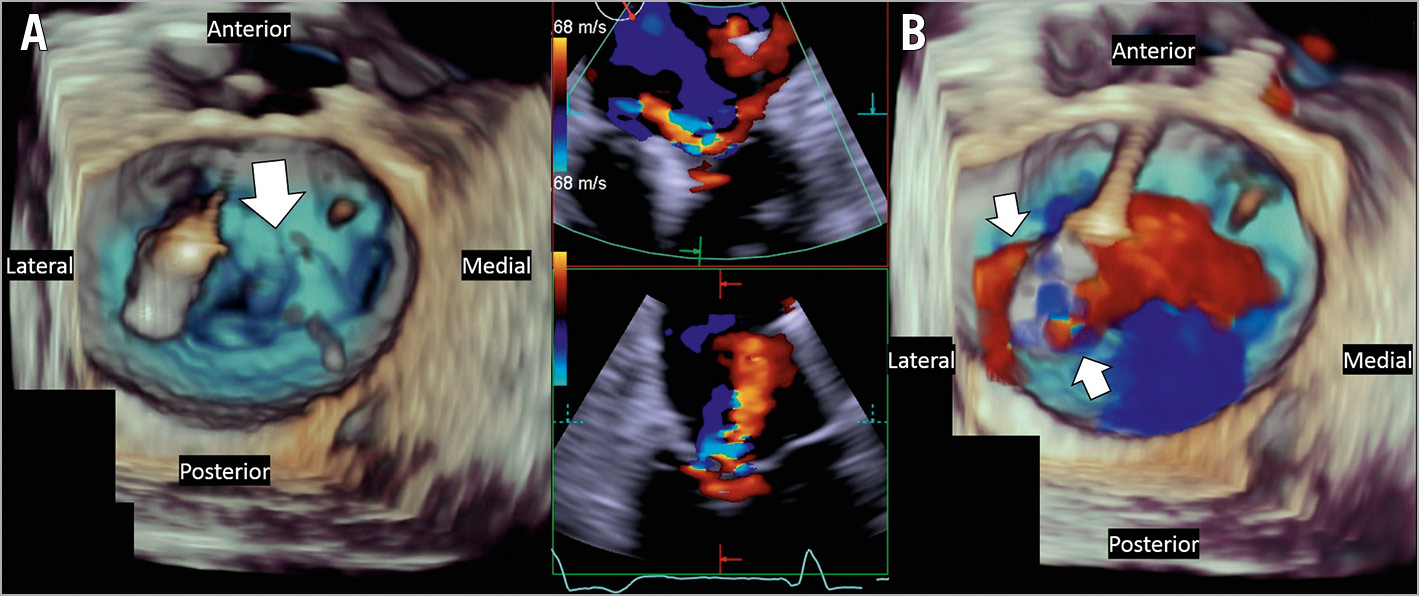
Figure 3. Example 3 was a patient with SMR complicated by posterior leaflet tear. A medial clip (XTR) was initially placed laterally and then repositioned medially (3 grasping attempts, final position, arrow in panel A). Multiple attempts at a second, lateral clip (XTR) resulted in a tear in the posterior leaflet and 4+ MR (B), which was also noted in the surgical report (surgery done 6 days after TMVr). The patient died postoperatively (13 days post TMVr) from right ventricular failure and multiorgan dysfunction.
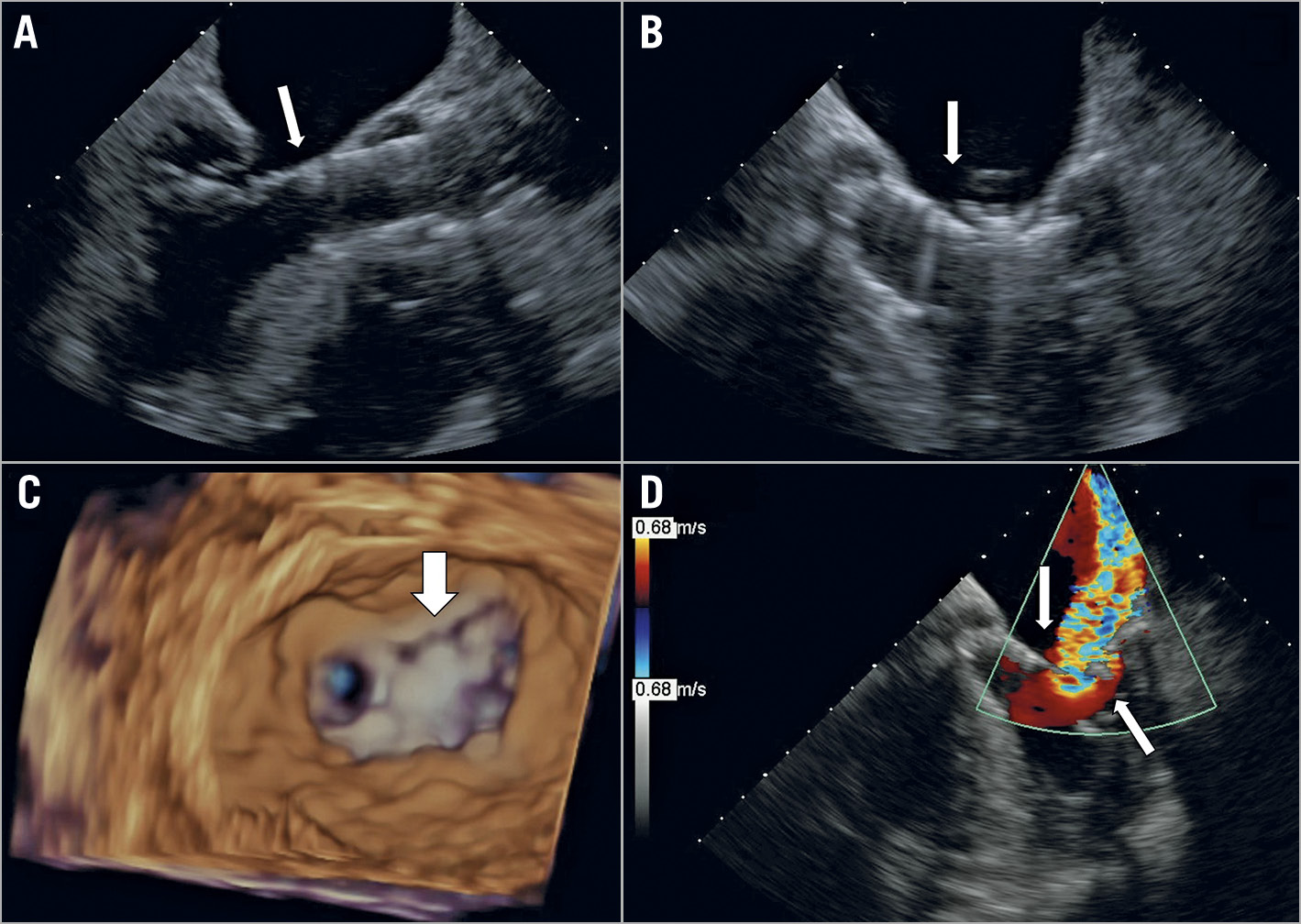
Figure 4. Example 4 had severe aortic stenosis and severe MR of mixed aetiology including significant thickening of the anterior MVL and chordae, complicated by leaflet tear. Initially, TAVR was performed and was followed with MitraClip implantation. A self-expanding TAVR was implanted low in the LVOT (A, long-axis view), which resulted in anterior leaflet impingement after the first clip (XTR, 2 grasping attempts) was deployed (B, arrow marks clip grasping at site of TAVR device). After a second clip (XTR, 5 gripping attempts), a tear in the anterior leaflet was noted (C, arrow) with residual 4+ MR (D, long-axis view with arrows marking the TAVR edge and clip, with a large jet in between them). No further intervention was carried out due to extreme high risk and the patient died 7 days later.
SINGLE LEAFLET DEVICE ATTACHMENT
Of the 24 patients reported as having SLDA by the enrolling centres, 14 were confirmed by the committee and 5 were ruled out; in 5 other patients the adjudication was inconclusive due to insufficient imaging and clinical data for the committee’s case review. The committee identified 4 additional SLDA events: 2 had SLDA alone and 2 had coexisting SLDA and leaflet injury (cases 1 and 2 described above), that were reported by the sites as leaflet injury. Overall, SLDA was confirmed in 18 patients (1.8% incidence). Sixteen of these patients had SLDA alone and 2 had coexisting SLDA and leaflet injury (cases 1 and 2 described above). The clip was detached from the posterior mitral valve leaflet (MVL) in 15 cases (83%), and from the anterior MVL in the remaining 3 cases (17%). Ten cases were related to XTR and 8 related to NTR. Four events were identified during the implant procedure (including the 2 with leaflet injury), 7 before hospital discharge and 7 at 30 days of follow-up (2 were reported by the sites at 6 months but upon review of the committee they were present in earlier echoes). Details of the 16 cases adjudicated as SLDA alone are described in Table 3; examples are shown in Figure 5. Among cases with SLDA alone (2 of which had double SLDA, both in cases of PMR), 5 (31%) had features of mitral valve complexity including 2 with complex degenerative leaflets with a wide gap, 2 with the jet outside A2/P2 and 3 with small valves, calcified landing zone and/or minimum leaflet tissue for grasping. Residual MR after the procedure was 3+ or 4+ in 4 cases (25%), 2+ in 5 (31%) and 1+ or less in the remaining 7 (44%). Serious adverse events occurred in 4 cases (25%): 1 death, 1 stroke and 2 cases that required cardiac surgery.
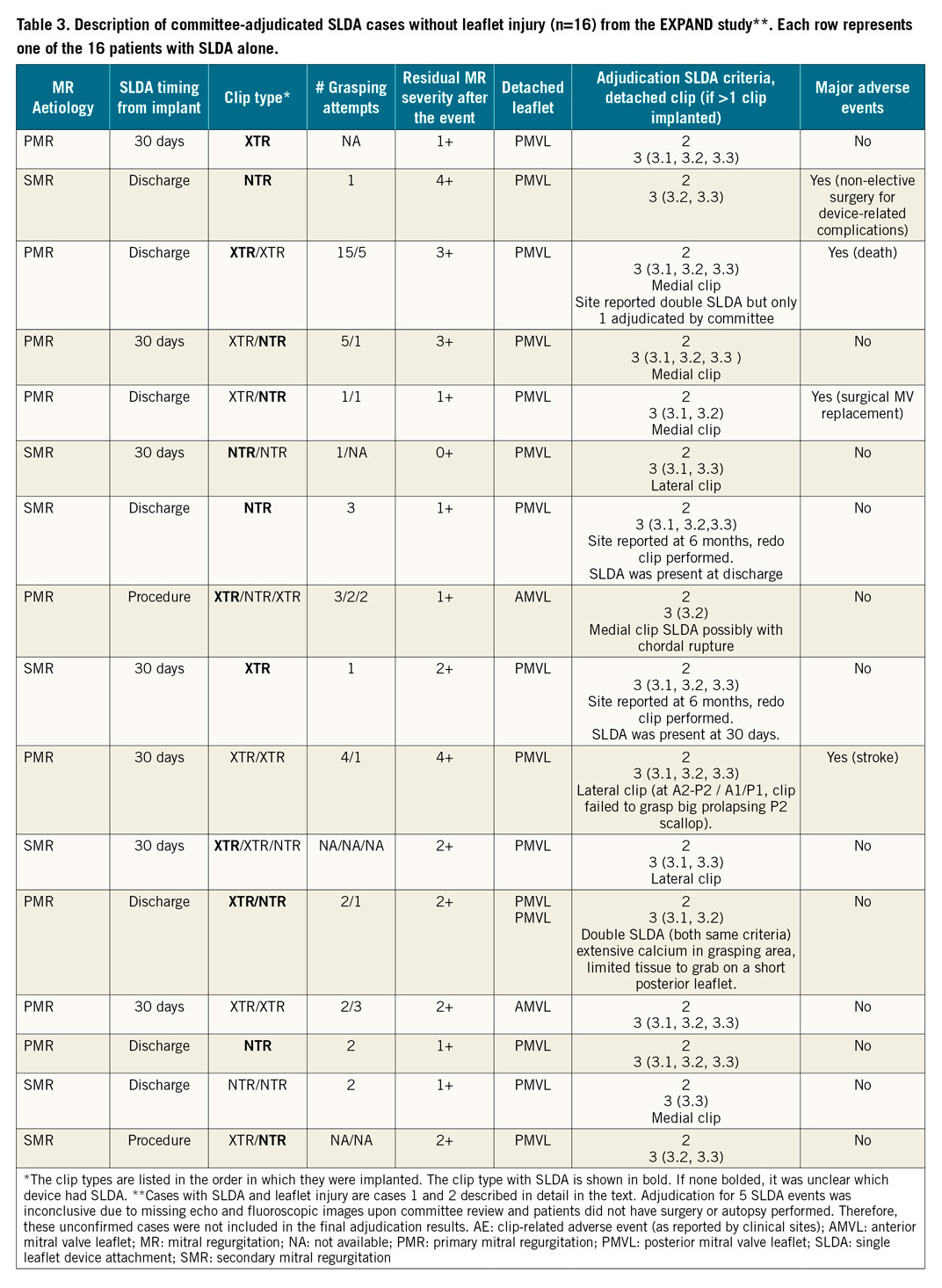
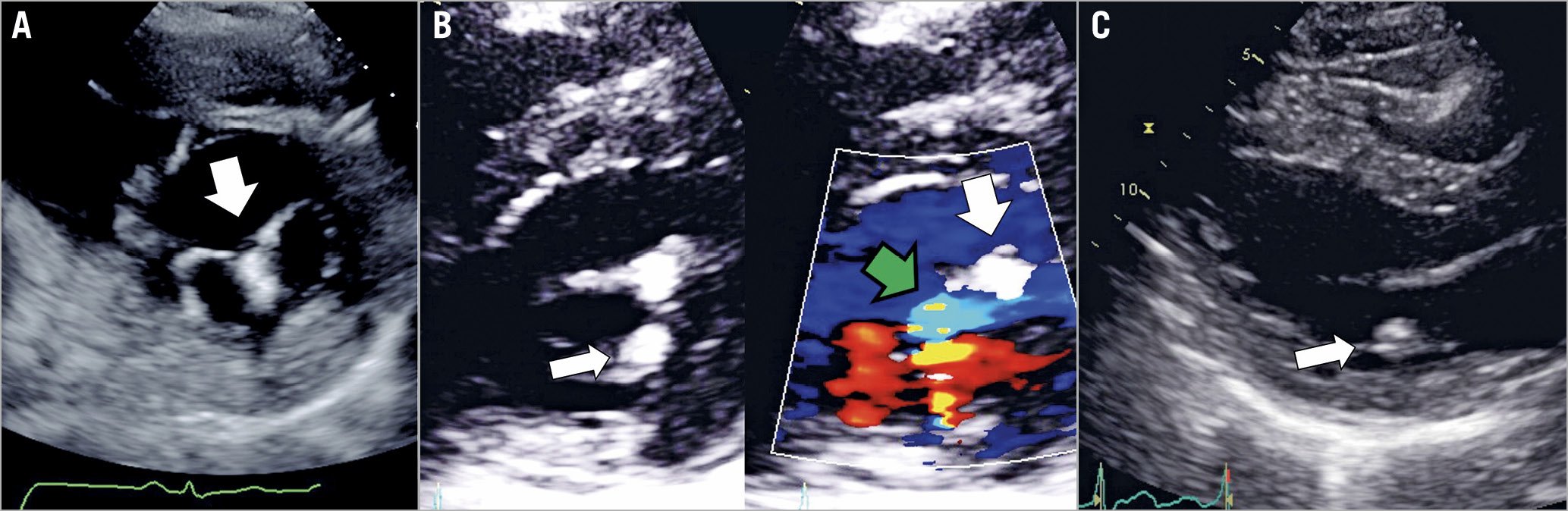
Figure 5. Single leaflet device attachment. A) A short-axis view of a normal clip position in A2/P2, attached to both leaflets and creating a diastolic bridge that gives the mitral valve an “8” shape (arrow). B) (short-axis) & C) (long-axis). A case of SLDA, where a medial clip is attached only to the posterior leaflet (narrow arrows). Note the loss of the diastolic bridge and a jet of colour seen between the clip and the detached anterior leaflet (green arrow), while a lateral clip is properly attached to both leaflets (wide white arrows).
The proposed SLDA diagnostic criterion 2 – complete separation of clip and leaflet – was present in all 16 cases. All three features of criterion 3 (3.1 lack of diastolic bridge, 3.2 jet between device and leaflet, and 3.3 excessive leaflet mobility) were present in 8 cases. Six cases had 2 features and 2 cases had a single feature (these were all confirmed – definite SLDA – on the basis of criterion 2). The site-reported suspected SLDA cases that were reviewed by the committee and not adjudicated as SLDA (n=5) had a common echocardiographic presentation of a small jet between the clip and leaflet, lacking complete clip/leaflet separation or without clear lack of diastolic tissue bridge.
Discussion
Over the last decade, the practice of edge-to-edge mitral valve repair with the MitraClip technology has expanded enormously worldwide. Meanwhile, the third-generation evolution of the MitraClip introduced NTR and XTR clip sizing options and an improved delivery catheter for ease of use. The XTR has longer clip arms (by 3 mm) and adds two additional rows of frictional elements per arm to distribute the load across the leaflet. The XTR clip design is aimed at improving grasping, coaptation area and overall ease of use, and, together with the improvements in the delivery system, resulted in greater MR reduction without an increase in adverse events11.
The expert panel in EXPAND performed the first comprehensive review on LAE associated with the MitraClip, adjudicating site-reported LAE and providing the first set of definitions and classification of different forms of leaflet injury and suboptimal device attachment. Our findings are as follows: 1) the use of novel standardised definitions allowed accurate systematic adjudication of LAE; 2) the overall incidence of LAE was low (2.0 %) and occurred similarly with NTR (in 9 cases) and XTR (in 12 cases); 3) LAE seemed to be related to multiple grasping attempts but not to mitral valve anatomical complexity and occurred both in PMR and SMR; 4) leaflet injury was rare and was significantly over-reported by the enrolling centres, occurred at the time of implant, resulted in severe MR and portrayed an ominous prognosis; 5) SLDA incidence was low, occurred either at the time of implant or during follow-up, and most cases could be resolved with subsequent deployment of additional clips.
Study sites reported 35 suspected LAE in 1,041 enrolled subjects, out of which 20 (2.0%) were confirmed as LAE by the expert committee. The committee was able to evaluate all events except 5 cases which could not be properly evaluated due to missing images. The final adjudication results indicate a low incidence of LAE (2.0%), and a low SLDA or leaflet injury event rate (4 leaflet injury [0.4%], 16 SLDA-alone [1.6%] events) and demonstrate the safety of MitraClip NTR and XTR systems in a multicentre, international study in contemporary real-world settings. The incidence of adverse events was comparable to previous generations of the MitraClip system. The TVT registry reported a 1.5% incidence of SLDA and a 0.8% rate of other device-related adverse events7. However, this US registry captured only procedural and in-hospital events and therefore may have under-reported later SLDA events that were captured in our clinical study (7 cases occurred after discharge in EXPAND). In ACCESS-EU, SLDA occurred in 4.8% of the cases and data regarding leaflet injury were not reported12. Of note, these two studies reflect early experience in clinical centres, involved earlier generations of the device and lacked systematic, independent analysis of the cases by an expert multidisciplinary panel. The results from our analysis suggest that, contrary to initial concerns, the third-generation MitraClip NTR and XTR systems are at least as safe as previous generations. It is important to note that the clinical centres participating in EXPAND were required to have experience with previous generations of the device and had completed at least three implant cases of the NTR/XTR systems prior to enrolling subjects into the study.
The agreement rate between the site and the committee assessment was high for SLDA. The committee adjudication results differed from the site reporting in only 5 cases. Leaflet injury, however, was over-reported by the sites and only confirmed in 4 of the 11 cases. This high disagreement rate reflects the lack of specific definitions and directives on how to diagnose LAE accurately and consistently at the study inception, and highlights the need for the agreed definitions introduced in this manuscript. The definitions for SLDA and a variety of leaflet injury categories will allow timely identification and consistent reporting of LAE in clinical trials and in clinical practice, and improve the evaluation of safety of the TMVr procedures. The LAE definitions provided by the expert panel based on the EXPAND study are novel and can potentially be improved further over time as the global experience with transcatheter edge-to-edge repair with the MitraClip and other TMVr techniques grows.
Based on the observations from the EXPAND study, leaflet injury occurred at the time of the implant, while SLDA occurred mostly during the implant or prior to hospital discharge (early events), and less often post-procedurally up to 30 days (late events). There were no reported LAE after 30 days up to one-year follow-up. Therefore, it is important to explore leaflet injury and SLDA when significant MR occurs during the implant procedure and to consider late SLDA in the event of worsening MR severity during follow-up.
Differential diagnosis between types of LAE is crucial for helping the decision-making process in the catheterisation lab. If an LAE happens intraprocedurally, the correct interpretation of the echocardiographic and fluoroscopic findings will be of paramount importance. The presence of a clip attached on one of the two leaflets often results in minimal challenges to the operator, as SLDA can be managed by an additional MitraClip implantation in most cases13. However, some SLDA cases can be more difficult to handle, particularly those with anatomical valve complexity such as significant leaflet or annular calcification, restricted valve opening, small leaflet tissue to grasp, commissural jets or presence of a large cleft. The review of site-reported events revealed that partial leaflet insertion, misalignment between the clip arms and leaflets and the associated residual jet can often be confused with SLDA. The agreed echocardiographic definitions provided herein should be used for the differential diagnosis of SLDA to identify the corrective action to reduce the regurgitant jet. While leaflet injuries are uncommon, they carry a worse prognosis and can rarely be treated without a surgical procedure14,15,16. Leaflet perforations cannot be treated by an additional clip implantation, but successful treatment of residual MR and perforations with a nitinol occluder has been reported in isolated cases, although there is a risk of device embolisation or inducing haemolysis due to residual leak17,18. Rarely, a small leaflet tear could potentially be amenable to correction with an additional clip implantation or an occluder19. However, the chances of achieving a good result are limited and patients will most likely require emergency valve replacement, as was the case in 3 of the 4 patients in our study. Our findings suggest that operators should avoid multiple grasping attempts, particularly when treating patients with frail mitral valve leaflets. Shape distortion of the leaflets is most frequently the result of a misalignment between the clip arms and leaflets during gripping. In case of non-central implantation, the misalignment can originate before crossing the valve leaflets, since the presence of the chordae under the annular level will prevent proper alignment after valve crossing. When a distortion is generated, resolution with additional clips is unlikely; therefore, safety checks with transoesophageal echocardiography are critical immediately after grasping and before releasing, as clips cannot be retrieved once released from the delivery system. Any newly directed jet prior to clip release should be a trigger to rule out misalignment of clip implantation as there is a low threshold for repositioning. Leaflet shape distortion could also be the result of chordal entrapment.
Limitations
While EXPAND was a prospective study, the retrospective analysis of echocardiographic images by the committee and the real-world nature of the study resulted in variability in the quality of echocardiographic acquisitions and hence certain measurements and views may not have been available for all patients. In particular, event-related images for 5 site-reported SLDA cases were not available for the committee’s adjudication. The authors acknowledge that the rate of SLDA can be between 1.8 and 2.2% if any of the 5 inconclusive cases were actual SLDA. Furthermore, it is possible that the implanted hardware (clips) precluded proper viewing of smaller leaflet tears or perforations that did not result in significant MR. However, the committee had access to every echocardiogram submitted by the sites for each case and performed an independent analysis of the images that was then complemented by the individual procedural reports to arrive at agreed conclusions.
Enrolment was based on site interpretation of MR severity and anatomy rather than prospective core lab adjudication as was the case in randomised controlled studies such as COAPT, MITRA FR and EVEREST II2,3,4. While this resulted in enrolment of some patients considered by the core lab to have only moderate MR, it also included a significant number of cases with characteristics of “high-risk anatomy” that may have been excluded from other clinical trials. Despite the inclusion of such patients, our analysis of the EXPAND study proves the safety of the third-generation MitraClip NTR and XTR systems. Finally, while this is the longest follow-up report of MitraClip safety and leaflet events to date, longer-term events have not been recorded in this study.
Conclusions
TMVr with third-generation MitraClip NTR and XTR systems is safe and provides more sizing options for percutaneous treatment of MR than previous generations. The rate of adverse events is lower than previously reported in large registries with older generations. As implantation of MitraClip NTR and XTR systems continues to rise worldwide, increasing the awareness and proper diagnosis of significant LAE is timely and of critical importance. This study presents the first comprehensive review on leaflet-related adverse events associated with the MitraClip by a multidisciplinary expert physician panel. The analysis reports the full adjudication of the leaflet adverse events on the next-generation MitraClip NTR and XTR systems. The echo findings reported in this study will help to differentiate leaflet injury events from inadequate leaflet insertion and SLDA, and provide guidance for consistent diagnosis of leaflet events with the MitraClip.
|
Impact on daily practice Mitral valve repair with third-generation MitraClip NTR/XTR systems is safe for percutaneous treatment of mitral regurgitation. The rate of adverse events is lower than previously reported in large registries using older generations. |
Acknowledgements
The authors acknowledge Onur Dur and Kartik Sundareswaran, from Abbott, for their support in coordinating activities related to the independent Leaflet Adverse Events Review Committee. The authors also want to acknowledge all sites and study principal investigators who contributed to the EXPAND study.
Funding
The EXPAND study was funded and sponsored by Abbott, Santa Clara, CA, USA. ClinicalTrials.gov Identifier: NCT03502811
Conflict of interest statement
F. Asch has no personal disclosures. His work as Director of an academic core laboratory is through institutional research grants (MedStar Health) with Abbott, Boston Scientific, Medtronic, Edwards Lifesciences, Neovasc, Ancora Heart, LivaNova, MVRx, InnovHeart, Polares Medical, and Aria CV. S. Little has ongoing research support disclosures from Siemens Healthcare, and previous consultant roles for Abbott and Medtronic. G.B. Mackensen has been an unpaid consultant for Abbott. P. Grayburn has received research grants from Abbott Vascular, Boston Scientific, Cardiovalve, Edwards Lifesciences, Medtronic, Neochord, and W.L. Gore, and has been a consultant or advisory board member for Abbott, Edwards Lifesciences, Medtronic, W.L. Gore, and 4C Medical. P. Sorajja has been consulting for Abbott, Medtronic, Boston Scientific, Anteris, W.L. Gore, Neovasc, Vdyne, TriFlo, TriCares and Teleflex, has received research grants from Abbott, Medtronic, and Boston Scientific, and holds equity in Anteris. M. Rinaldi has been teaching courses and has been a speaker, consultant and proctor for Abbott and Edwards Lifesciences, has been an advisory board member and speaker consultant for Boston Scientific. F. Maisano has received grant and/or institutional research support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, NVT, and Terumo. He has received consulting fees, honoraria personal and institutional from Abbott, Medtronic, Edwards Lifesciences, Xeltis, and Cardiovalve. He has received royalty income/IP rights from Edwards Lifesciences. He is a shareholder (including share options) of Cardiogard, Magenta, SwissVortex, Transseptal Solutions, Occlufit, 4Tech, and Perifect. S. Kar has received grants and institutional research support from Abbott, Boston Scientific and Edwards Lifesciences, and consulting fees/honoraria from Abbott, Boston Scientific, W.L. Gore and Medtronic.
Supplementary data
To read the full content of this article, please download the PDF.

