
Abstract
Over the past decade, different transcatheter repair techniques have been developed to treat mitral regurgitation (MR) with less invasive approaches in order to address the unmet clinical need of untreated patients with MR. The aim of this report is to provide an overview of the currently available transcatheter mitral repair options, focusing on the evidence reported so far.
Abbreviations
DMR: degenerative mitral regurgitation
FMR: functional mitral regurgitation
LV: left ventricle
MR: mitral regurgitation
MVARC: Mitral Valve Academic Research Consortium
TMVI: transcatheter mitral valve implantation
Introduction
THE CLINICAL PROBLEM
Mitral regurgitation (MR) is a common entity which affects about 10% of individuals over 75 years of age in the general population1. The natural history of severe MR is unfavourable, leading to worsening LV failure, pulmonary hypertension, atrial fibrillation, and death2. The most common aetiology of primary MR in Western countries is degenerative MR (DMR), due to leaflet tissue alteration and degeneration, leading to mitral valve (MV) prolapse or flail3.
On the other hand, secondary or functional MR (FMR) is the consequence of left ventricular (LV) dysfunction and dilation, usually due to post-ischaemic or idiopathic dilated cardiomyopathy, leading to secondary MR4 due to annular dilatation and leaflet tethering, which prevent proper closure of the valve in spite of anatomically normal valve leaflets5.
Open heart surgery is the gold standard for the treatment of severe DMR as excellent outcomes can be achieved in most patients, often adopting minimally invasive approaches6.
In severe DMR in particular, surgical repair represents the optimal treatment. If performed before the onset of limiting symptoms, atrial fibrillation or development of LV dysfunction, MV repair is able to restore normal life expectancy and quality of life7. By contrast, surgical correction of functional FMR is controversial, since the prognosis is more related to the cardiomyopathic process than to MR itself. Outcomes after surgical correction of FMR remain suboptimal, recurrence of MR is frequent, and perioperative mortality is not negligible8-10.
Nevertheless, the vast majority of patients with MR do not undergo surgical treatment. The Euro Heart Survey conducted by the European Society of Cardiology (ESC) showed that up to 50% of patients with severe MR are denied surgical treatment because they are felt to be at too high risk for surgery owing to advanced age or comorbidities11. Other common reasons are a combination of limited local access to surgeons with expertise, general reluctance for an open procedure, or a lack of perceived survival benefit, especially in FMR12,13.
Therefore, over the past decade, different transcatheter techniques have been developed to treat MR with less invasive approaches, in order to address the unmet clinical need of untreated patients with MR. The field of percutaneous MR treatment is highly active, with several technologies being actively developed (including repair and replacement technologies) and some of the repair devices being already commercially available14.
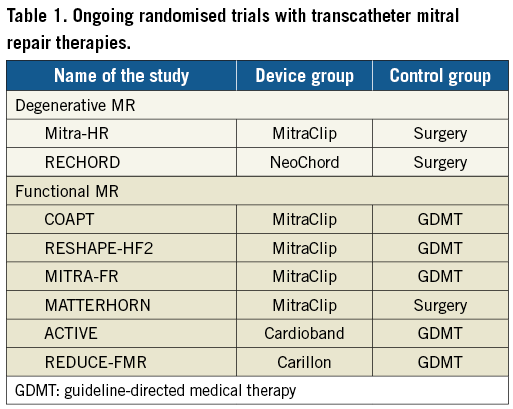
The aim of this particular report is to provide an overview of the currently available transcatheter mitral repair options, focusing on the (little) evidence reported so far. Table 1 shows the randomised trials currently recruiting in the field of transcatheter mitral valve repair.
THE COMPLEXITY OF THE MITRAL VALVE APPARATUS
From the beginning, it was clear to all physicians approaching the field of transcatheter MV treatment that addressing the MV would be associated with technical challenges and anatomical complexity in a totally different way compared to those of the aortic valve field15.
The MV is a complex apparatus integrated in the LV, including the annulus, the leaflets, the chordae, the papillary muscles, and the ventricle itself. Beyond its haemodynamic function to ensure forward cardiac output, the mitral apparatus plays a fundamental role in the structural and functional integrity of the LV; the discontinuation of this integrity is associated with worsening LV performance.
Abnormalities of any part of the MV complex (leaflets, annulus, chordae tendineae, and papillary muscles) or the LV can lead to MR. Not infrequently more than one component of the complex is affected. This represents a major challenge in terms of percutaneous repair, since it may be necessary for a single operator to master different techniques if a complete repair is the aim16.
The complexity of the subvalvular apparatus and the distribution of chordae tendineae increase the risk of damage or entrapment with all of the leaflet repair devices (Figure 1). The mitral annulus is a 3D saddle shape and a highly movable structure, which usually loses its 3D configuration and contractility in diseased valves. Different vital structures are in proximity to the mitral annulus, such as the coronary sinus and left circumflex artery, and thus are vulnerable to impingement by some devices.

Figure 1. View of the mitral valve apparatus from the LV. In this anatomical cut, the right ventricle and the interventricular septum have been removed in order to show the subvalvular apparatus of the MV and the distribution of the chordae tendineae. Each papillary muscle provides chordae for the anterior and posterior mitral valve leaflet. The green ellipse marks the “chordal-free zone”, which corresponds to the A2-P2 region (middle part of the mitral valve), where the risk of chordal impingement is minimal. The red ellipses show the regions corresponding to the lateral (A1-P1) and the medial (A3-P3) regions, where the risk of chordal impingement is higher. Chordal density is maximal in the commissural zones. AML: anterior mitral leaflet; APM: anterolateral papillary muscle; AV: aortic valve; PPM: posteromedial papillary muscle
TRANSCATHETER MITRAL VALVE REPAIR
As discussed above, the variety of different pathological mechanisms underlying MR and the complex anatomy of the mitral valve have led to the development of different technologies, each of them focused on the treatment of a specific anatomical lesion or component of the valve apparatus.
The latest 2017 European Society of Cardiology guidelines reflect the lack of evidence produced so far in order to support the clinical use of transcatheter mitral repair methods. In fact, the only recommendation for any transcatheter repair is a class IIb recommendation for transcatheter edge-to-edge repair, which may be considered in inoperable or high-risk patients with symptomatic severe MR, avoiding futility in this patient group with many comorbidities. However, it is interesting to note that this recommendation shares the same level of evidence (IIb) as mitral surgery in the context of isolated FMR, reflecting the paucity of evidence for surgical repair in this setting also17,18.
Transcatheter MV repair devices are conventionally classified into three groups: leaflet devices (including chordal repair), annuloplasty repair devices, and LV remodelling devices, according to the therapeutic target. To complete the portfolio of transcatheter mitral therapy, transcatheter mitral valve implantation (TMVI) is rapidly emerging (Figure 2, Figure 3).
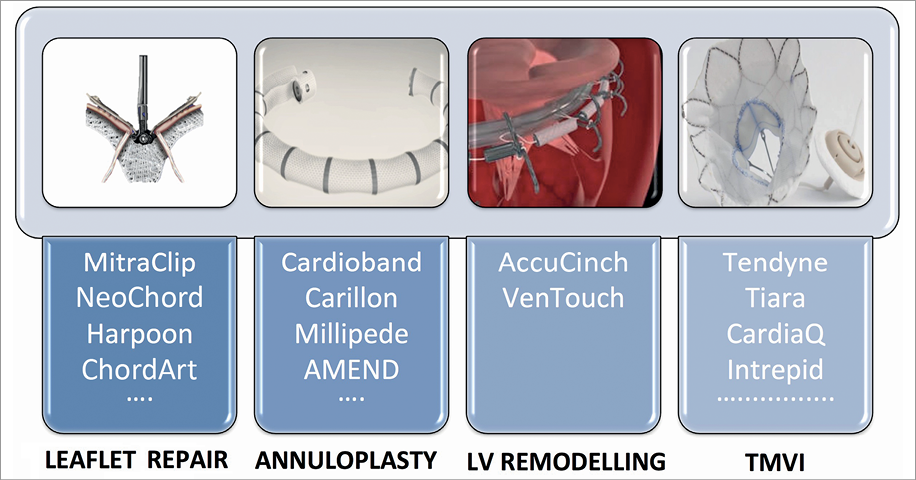
Figure 2. Classification of transcatheter mitral valve therapies according to the therapeutic target. TMVI: transcatheter mitral valve implantation

Figure 3. Transcatheter mitral valve repair devices. 1) MitraClip. 2) PASCAL. 3) Carillon. 4) NeoChord. 5) Cardioband. 6) AMEND. 7) Millipede. 8) Harpoon. 9) Carillon® Mitral Contour System. 10) ARTO. 11) VenTouch. 12) AccuCinch.
LEAFLET DEVICES
MITRACLIP® (ABBOTT VASCULAR, SANTA CLARA, CA, USA)
MitraClip implantation is the most widely available percutaneous therapy for native MR, with more than 60,000 patients treated worldwide so far. The main advantages of the MitraClip device are its versatility (since it has been successfully used in both degenerative and functional aetiologies and in different clinical settings) and its safety, which has been shown even in extreme risk heart failure patients19-23. Using a 24 Fr transvenous, transseptal system, one or more clips are used to approximate the free edges of the anterior and posterior leaflets, replicating by a percutaneous approach the so-called Alfieri edge-to-edge surgical mitral repair technique. The safety profile of MitraClip therapy is excellent. The in-hospital mortality rate reported in different series, including high-risk and inoperable patients and often including data from low-volume centres participating in the registries, is about 2-3%; the morbidity rate is also low. The duration of hospital stay in many series is less than three days. The rate of single leaflet detachment is low, now being 1-2%, while clip embolisation remains extremely rare13,19,20,22,24.
In terms of efficacy, procedural success (reduction in MR to ≤2+) occurs in about 90% of selected patients, with about 65% of patients having residual MR ≤1+19-21,23. Despite the improvement in intraprocedural imaging and patient selection, there is still a non-negligible proportion of patients with residual or recurrent significant MR. Limited information is available so far concerning the long-term durability of MitraClip therapy, especially in patients with a functional aetiology25.
Final five-year results of the randomised EVEREST II trial showed that MitraClip therapy was less effective in reducing MR than open surgical repair in standard surgical candidates, mainly with DMR26. Currently, the MitraClip is approved for treatment of high-risk patients in Europe (primary or secondary MR) and in the USA (primary MR only), with a class IIb recommendation17,18.
Despite current European guidelines recommending the use of MitraClip for both functional and primary mitral regurgitation, differences in reimbursement policies across European countries have resulted in different usages (e.g., in France, MitraClip is reimbursed only for primary MR).
A randomised trial in France comparing MitraClip with surgical repair in high-risk but operable patients with DMR has just started enrolment, namely the Mitra-HR trial (ClinicalTrials.gov Identifier: NCT03271762). MitraClip use in real-world clinical practice is mainly reserved for high-risk candidates with FMR. The acute safety and efficacy results are similar for both aetiologies, although the one-year mortality is, unsurprisingly, higher for FMR patients.
Risk factors for suboptimal outcome and increased mortality after MitraClip therapy include residual or recurrent significant MR >2+, severe tricuspid regurgitation or right ventricular dysfunction at baseline, severe pulmonary hypertension and advanced heart failure19,22,24,27,28.
Although symptom improvements, reduction in HF hospitalisation and LV positive reverse remodelling have all been shown after MitraClip treatment, a survival benefit has not been demonstrated as yet. Ongoing randomised trials (COAPT, RESHAPE-HF2 and MITRA-FR) comparing MitraClip and optimal medical therapy will probably address this clinical issue.
The new MitraClip XTR has recently been introduced, with longer clip arms and improved device steerability.
Recently, the potential cost-effectiveness of the MitraClip as compared to surgery or medical therapy has been reported. Feasibility of early discharge after the procedure could potentially contribute further to improving the cost-effectiveness of MitraClip therapy, mainly in heart failure patients29-31.
PASCAL MITRAL VALVE REPAIR SYSTEM (EDWARDS LIFESCIENCES, IRVINE, CA, USA)
The PASCAL device (PAddles Spacer Clasps ALfieri) is a new technology to perform transcatheter edge-to-edge mitral repair. Introduced with a 22 Fr steerable system through the transseptal approach, it consists of a 10 mm central spacer that acts as filler in the regurgitant orifice, and is attached to the valve leaflets by two paddles and clasps. The clasps can be operated either simultaneously or independently to facilitate leaflet capture in complex anatomies.
First-in-man experience in 23 patients with severe MR and thought not to be good anatomic candidates for MitraClip therapy (EuroSCORE II 7.1%, STS score 4.8%) saw the device successfully implanted in all the cases, resulting in a procedural residual MR of grade 2+ or less in 22 (96%) patients. The safety profile was favourable, with only two observed minor periprocedural complications (one minor bleeding event and one transient ischaemic attack). M-VARC technical success was achieved in 22 out of 23 patients (96%), and device success at 30 days was achieved in 18 (78%) patients. Three patients (13%) died during the 30-day follow-up. Significant clinical improvements were observed in the 20 patients alive 30 days after implantation32.
Based on surgical experience, the main limit of both transcatheter edge-to-edge approaches is the absence of annuloplasty, which may affect durability of the repair at long-term follow-up. The use of combined therapy (leaflet+annuloplasty devices) can potentially overcome this limit and provide better durability33.
NEOCHORD DS1000 (NEOCHORD, ST. LOUIS PARK, MN, USA)
Based on a solid surgical background, catheter-based implantation of the NeoChord is an appealing option to treat DMR. The NeoChord DS1000 is a minimally invasive system that uses adjustable PTFA sutures, through a transapical approach, on the beating heart, to enable support to the free edge of MV leaflets with real-time adjustment of the artificial chordal length for treatment of DMR.
Following the initial results reported in the TACT feasibility trial, with an overall reduction of MR to ≤2+ in 86.7% of 30 patients, the device obtained CE mark in 2012 and has been used in over 500 patients so far34.
The efficacy in reducing MR clearly depends on the complexity of the lesion. In simple anatomy, such as isolated P2 segment flail or prolapse, one-year freedom from a composite endpoint including mortality, MR recurrence, mitral surgery, rehospitalisation and stroke was 94%. In the presence of multiple posterior leaflet segment prolapse/flail or in the presence of a more complex anatomy (bileaflet or commissural involvement), freedom from the composite endpoint was 82% and 63%, respectively. A learning curve effect has also been observed35,36.
In the USA, NeoChord is currently under clinical investigation in the “Randomized trial of the NeoChord DS1000 system versus open surgical repair” (RECHORD). The major limitation of this technology with the current-generation device is the transapical access. The device is delivered sheathless and requires multiple entries and exits through the apex in case of the need for multiple chordae, with a consequent bleeding risk and the challenge of treating multi-segment disease.
HARPOON TSD-5 (EDWARDS LIFESCIENCES)
Recently, the Harpoon device has been investigated in an early feasibility trial in patients with DMR and posterior leaflet prolapse. The Harpoon system is a 14 Fr device that allows the operator to implant multiple PTFE chordae through the transapical access, on the beating heart, and subsequent live transoesophageal echocardiography (TEE) adjustment of the chordal length. It differs from the NeoChord in terms of its leaflet engaging mechanism (leaflet anchoring with a preformed knot instead of free-edge leaflet fixation) and the use of a smaller introducer sheath on the LV apex which allows easier and repeated access. The fixation of the neochordae far from the free edge of the leaflet may represent a concern in long-term follow-up.
Among the 30 patients reported in the initial feasibility trial, three patients required conversion to open mitral surgery. There were no deaths, strokes, or permanent pacemaker implantations. At one month, MR was mild or less in 89% (24/27) and was moderate in 11% (3/27). At six months, MR was mild or less in 85% (22/26), moderate in 8% (2/26), and severe in 8% (2/26). Favourable cardiac remodelling at six months including decreases in LV and left atrial (LA) volumes, as well as mitral annulus diameter reduction, was observed37.
Patient selection remains the critical point to both chordal implantation devices. So far, the ideal candidate for a successful and durable repair seems to be the patient with a monosegmental DMR, no or mild annular dilatation and limited LV dilatation (due to the risk of recurrent prolapse in case of LV volume reduction). Moreover, differently from surgical chordal repair, the distal extremity of the PTFE suture is not fixed to the papillary muscles, as in the native anatomy, but is fixed to the apical access. The potential consequences of this last aspect at long-term follow-up are unknown.
CHORDART™ (COREMEDIC GMBH, TUBINGEN, GERMANY)
In order to overcome some of the limitations of the two abovementioned devices, the ChordArt system has been developed to implant pre-measured neochordae by using an antegrade approach (transatrial or transseptal), with a unique and simple method of anchoring the distal chord directly to the papillary muscle (therefore in its anatomical position). The size of the implant can be established on echo or computed tomography (CT) preoperative imaging. The device is completing preclinical validation and FIM implant is planned by Q1 of 2019 (Weber A. Changing the Way of Treating Mitral Valve Prolapse: A Sutureless Neochordal Replacement. TCT 2016, Washington, DC, USA. Available at https://www.tctmd.com/slide/changing-way-treating-mitral-valve-prolapse-sutureless-neochordal-replacement).
Similar to the edge-to-edge approach, all the transcatheter chordal repair approaches are carried out without mitral annuloplasty. This could potentially have a negative impact on the long-term durability of the repair.
ANNULOPLASTY DEVICES
Mitral annuloplasty is the most commonly performed technique during conventional mitral surgery, in order to restore the normal ratio between the leaflet surface area and the annular area, to improve leaflet coaptation, and to counteract progressive annular dilatation that can lead to recurrent MR. Surgical annuloplasty is almost invariably performed in conjunction with leaflet repair in DMR treatment and it is commonly used as stand-alone treatment in selected patients with FMR, in order to force leaflet coaptation using undersized annuloplasty38,39. Suture annuloplasty techniques have almost been abandoned in surgical practice, with the availability of the different ring prostheses that are implanted on the atrial surface of the mitral annulus. A number of prosthetic rings are commercially available, with different features for different anatomical and clinical scenarios (complete versus incomplete rings, flexible versus semi-rigid versus rigid, aetiology-specific rings); however, operator preference and experience is the main factor determining the choice of ring in most cases.
Transcatheter annuloplasty has the potential to serve as a stand-alone therapy in selected patients with FMR with a minimal anatomical and physiological impact, with the advantage, differently from the edge-to-edge devices for example, of keeping the option open for eventual further leaflet treatment or for mitral valve implantation. Moreover, it has the potential to be associated with leaflet repair in order to improve the durability of the repair, or to serve as a future platform for valve-in-ring implantation.
Different percutaneous annuloplasty approaches have been developed to remodel the mitral annulus either indirectly (based on the anatomic proximity of other structures, such as the coronary sinus, to the posterior mitral annulus) or directly, using anchors or sutures to implant the device on the atrial surface of the mitral annulus, to perform either a partial or a complete mitral annuloplasty.
INDIRECT ANNULOPLASTY
CARILLON® MITRAL CONTOUR SYSTEM (CARDIAC DIMENSIONS, KIRKLAND, WA, USA)
The Carillon device is an indirect annuloplasty device delivered by the transjugular approach. It consists of two self-expanding nitinol anchors connected by a nitinol curvilinear segment (different lengths are available), which are implanted in the coronary sinus, in close proximity to the posterior annulus of the MV. Carillon obtained CE mark in 2011. The TITAN trial evaluated the clinical impact of the Carillon in heart failure subjects with significant FMR and showed a significant reduction in FMR grade (effective regurgitant orifice area [EROA] 0.23±0.07 cm² to 0.12±0.08 cm² and regurgitant volume 34±10 ml to 17±12 ml) at one-year follow-up40.
Treated subjects were compared with a pseudo-control group consisting of subjects without implants. Functional and performance status significantly improved in the treated subjects41. The REDUCE FMR trial, a blinded, randomised (3:1) sham-controlled trial in about 180 patients, has completed enrolment in Europe, Australia and New Zealand and will probably be reported before the end of 2018. A pivotal trial in the USA in which patients with secondary MR will be randomised 2:1 to receive the device versus optimal medical therapy is soon to be underway.
Some anatomical limitations explain why this approach cannot be effective in a relevant proportion of patients with FMR. First of all, the coronary sinus and mitral annulus are not coplanar. Moreover, depending on the individual coronary anatomy, there is a risk of coronary artery compression that may preclude patient eligibility. The main advantages are that the procedure is technically easy to perform, can be carried out under local anaesthesia and in centres without on-site cardiac surgery, and is safe.
ARTO™ SYSTEM (MVRx, INC., SAN MATEO, CA, USA)
The ARTO is an indirect annuoloplasty device that consists of a suture connecting two anchors, one implanted in the interatrial septum and one in the coronary sinus. The tensioning of the connecting suture shortens the anteroposterior diameter of the mitral annulus, resulting in a reduction of the anteroposterior mitral annular diameter and improved leaflet coaptation.
In the early feasibility MAVERIC trial, 11 high-risk patients with FMR grade ≥2+ underwent successful ARTO System implantation, with no procedural safety events. Six-month follow-up showed improvements in MR grade (about 80% of the patients with MR ≤2, PISA from 30.3±11.1 mm² to 13.7±8.6 mm²), as well as in functional status42. Phase II trial enrolment is ongoing.
The main concern regarding indirect annuloplasty is the total lack of surgical background.
DIRECT ANNULOPLASTY
CARDIOBAND™ (EDWARDS LIFESCIENCES)
The Cardioband is the transcatheter device closest to a surgical ring and is the direct annuloplasty system with the widest clinical experience so far, having been implanted in about 500 patients with FMR. It is delivered from a fully percutaneous transseptal approach and the implant is performed on the atrial side of the mitral annulus. An incomplete adjustable surgical-like Dacron band is implanted from the lateral to the medial trigone, under live echo and fluoroscopic guidance, using multiple retrievable 6 mm anchor elements. The size of the band is determined by preoperative angio-CT assessment, which is performed also to rule out the presence of annular calcifications (the main anatomical contraindication to this procedure) and the proximity of the circumflex coronary artery. After implantation, the Cardioband length may be shortened on the beating heart under live echo guidance to reduce the anteroseptal diameter of the mitral annulus, improve leaflet coaptation and reduce MR.
The Cardioband obtained CE mark approval at the end of 2014. Results from the early feasibility trial (30 high-risk patients with significant FMR) showed a procedural success rate of 100%, with no periprocedural deaths and a 30-day mortality of 5%. Adjustment of the implanted Cardioband reduced the annular septolateral dimension by >30% (from 3.7±0.5 cm at baseline to 2.5±0.4 cm after one month and to 2.4±0.4 cm after six months). Residual MR was ≤2+ in 86.3% of patients at six-month follow-up. Significant improvement in NYHA class, functional status and quality of life was also reported43,44. Limited late follow-up suggests that MR reductions may be durable. The partial design of the band may represent an issue in terms of long-term durability, since usually in surgery complete prosthetic rings are used in the context of FMR. However, the reduction in septolateral dimension of about 30% observed in the trial is absolutely comparable with the one obtained in surgery after undersized annuloplasty with complete rings.
IRIS TRANSCATHETER ANNULOPLASTY RING (MILLIPEDE MEDICAL, SANTA ROSA, CA, USA)
The IRIS annuloplasty system is a semi-rigid complete adjustable ring with a nitinol frame, which is placed on the atrial surface of the mitral annulus, via the transseptal approach, by means of multiple anchor elements. Successful first-in-human transseptal implantation was announced in May 2017 45.
AMEND™ (VALCARE MEDICAL, HERZLYIA PITUACH, ISRAEL)
The AMEND is a complete, semi-rigid, D-shaped mitral ring, which is implanted on the atrial side of the mitral annulus by means of 12 anchors, through the transapical access. Successful first-in-man implantation has recently been reported, and a fully percutaneous transseptal device is under preclinical evaluation46.
Being a complete annuloplasty device, it can potentially serve as a platform for future valve-in-ring implantation.
MITRAL RESTRICTION RING (CARDIAC IMPLANT SOLUTIONS LLC, JACKSONVILLE, FL, USA)
The Mitral Restriction Ring is another direct annuloplasty device delivered via the transseptal route, which is currently under preclinical investigation. It allows the implantation of a complete adjustable mitral ring with an internal cinching wire on the atrial annular side by means of multiple anchor elements. The implantable actuator is designed to enable non-invasive chronic progressive cinching at follow-up, following the completion of tissue healing (KH Kuck. Cardiac Implants Direct Annuloplasty for Functional MR: Device, Procedure, and Outcomes. TCT 2017, Denver, CO, USA. Available at https://www.tctmd.com/slide/cardiac-implants-direct-annuloplasty-functional-mr-device-procedure-and-outcomes).
LV REMODELLING DEVICES
To address ventricular abnormalities as the underlying cause of MR, the VenTouch system and the AccuCinch have been developed as ventricular reshaping devices.
The VenTouch™ system (Mardil Medical, Minneapolis, MN, USA), which was first implanted in humans in 2014, consists of a bladder that is positioned over the posterior-septal lateral aspects of the LV using a left thoracotomy, and inflated to reduce the dimensions of the LV and the mitral annulus, improving leaflet coaptation12,47.
The AccuCinch® (Ancora Heart, Santa Clara, CA, USA) is a device consisting of a series of nitinol anchors that are deployed subvalvular on the ventricular side of the mitral annulus, using a transfemoral, retro-aortic approach. A tension cable enables adjustment and consequent ventriculoplasty on the heart, resulting in MR reduction. The AccuCinch is currently under investigation in early feasibility human studies48.
The proof of concept for ventricular reshaping was suggested by the randomised RESTOR-MV trial, in which a tether implanted through the LV cavity resulted in chamber remodelling and decreased MR, with a positive impact on patient survival49.
CURRENT CHALLENGES AND FUTURE PERSPECTIVES
At the moment, the decision between the different approaches is mainly based on operator experience, anatomic exclusions for the different devices, and availability of the different technologies, since comparative evidence to support one specific device or category over another in the different clinical settings simply does not exist.
If we assume that transcatheter mitral interventions are the natural evolution of modern mitral valve surgery, the indications may continue to move from a palliative target (improving symptoms, treating advanced and end-stage disease) towards the aim of improving prognosis by an early indication. Early results show a high safety profile for these procedures, but effectiveness requires further study.
Improvement of advanced imaging for patient screening and procedural guidance will play a fundamental role in patient selection and optimisation of the outcomes. Another key point is the specific training of structural mitral operators, that should ideally include a common educational pathway with interventional, surgical and imaging knowledge.
Finally, it will be of extreme importance to collect proper evidence in a rigorous way, in order to support the different decisions in the different clinical scenarios. It is likely that transcatheter mitral repair will explode as TAVI did before, but this will take more time due to a greater clinical and technical complexity, keeping in mind that both the patient and the physician are always in the middle of this technological “revolution”50.
Conclusions
Several categories of device are currently available for transcatheter mitral valve repair. They are at different phases of clinical evaluation in a field which is expanding extremely rapidly. Transcatheter mitral valve replacement is quickly becoming clinically available, expanding the available portfolio, in the absence of clear indications or recommendations, for specific patient subsets.
Conflict of interest statement
Maurizio Taramasso is consultant for Abbott Vascular, Boston Scientific, 4tech; received personal fees from Edwards Lifesciences, Mitraltech, CoreMedic and Swissvortex; Shareholder of 4tech. Francesco Maisano discloses Grant and/or Research Support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific Corporation, NVT, Terumo, Consulting fees, Honoraria from Abbott, Medtronic, Edwards Lifesciences, Swissvortex, Perifect, Xeltis, Transseptal solutions, Cardiovalve (Mitraltech), Magenta , Royalty Income/IP Rights Edwards Lifesciences and is Shareholder of Cardiogard, Magenta, SwissVortex, Transseptalsolutions, Occlufit, 4Tech, Perifect. All other authors have no conflicts of interest to declare.

