
Mitral regurgitation: a clinical challenge
A 75-year-old patient was suffering from debilitating dyspnoea due to severe degenerative mitral regurgitation (MR) responsible for left ventricle enlargement. The Heart Team estimated that the patient was inoperable due to excessive surgical risk. The patient was treated with transcatheter percutaneous edge-to-edge intervention in accordance with current international guidelines1,2. After implantation of two clips, the transmitral mean gradient was 7 mmHg and residual MR was moderate. Should this patient have been considered for transcatheter mitral valve replacement (TMVR) instead of repair?
MR is present in >1% of the Western population over 70 years old, and is responsible for increased mortality risk in a similar fashion to aortic stenosis3. Transcatheter aortic valve implantation (TAVI) has allowed the treatment of patients presenting with aortic stenosis previously considered inoperable with tremendous success4. Since its introduction, the indications for TAVI have expanded and the technique is currently being investigated in all-comers (NCT02701283, NCT02675114). Conversely, MR is still undertreated5. The prohibitive surgical risk is unlikely to be the sole reason for MR undertreatment.
Limitations of transcatheter mitral valve repair
The MitraClip® (Abbott Vascular, Santa Clara, CA, USA) was the first transcatheter mitral valve intervention device to be developed. It aims to reproduce percutaneously the surgical Alfieri technique6,7. The popularity of surgical mitral valve repair at the beginning of the 21st century encouraged the development of numerous percutaneous mitral plasty devices8,9. The valvular plasty systems were later supplemented by the development of percutaneous annuloplasty (such as the Cardioband; Valtech Cardio, Or Yehuda, Israel) and chordal plasty (such as the NeoChord; NeoChord Inc., St. Louis Park, MN, USA) systems10,11. Nevertheless, after fifteen years of development, transcatheter mitral valve intervention is still struggling for broad adoption. A difficult learning curve as well as lack of efficiency on resolution of the mitral regurgitation are among the main limitations10.
The recent MITRA-FR and COAPT trials both showed that compassionate use of the MitraClip does not save patient lives, nor does it reduce their risk of rehospitalisation. However, very selected patients with secondary severe MR associated with a moderate left ventricle dysfunction and a suitable anatomy for implantation benefit from a reduced long-term mortality and rehospitalisation from heart failure risk if they have an interventional reduction of MR as an adjunct to optimal medical therapy12,13. The results of the RESHAPE-HF (NCT01772108, comparison against medical therapy) and MATTERHORN (NCT02371512, comparison against mitral surgery) trials might provide further insight into the appropriate use of the MitraClip for the treatment of secondary MR. However, it is worth noting that, despite extensive preoperative echocardiographic screening in COAPT, more than one clip was necessary in >60% of patients (≥3 clips in 8% of cases) to achieve satisfactory reduction of MR in the COAPT MitraClip (MC) group. This underscores the lack of efficacy of the device to achieve persistent low grade MR after the intervention. On the other hand, the implantation of an excessive number of clips can yield significant mitral stenosis in up to one fourth of patients, which has been reported to be associated with worse outcomes12,14.
It is worth remembering that the Alfieri technique has been described for primary MR. One of the reasons it was abandoned is the high rate of recurrence. In the original Alfieri technique cohort, the rate of reoperation was 10% at five years6. The five-year results of the EVEREST II trial reported a similar rate in its surgical (mostly repair) group15. MR is a multifactorial disease that could be due to a primary valvular dysfunction such as prolapse (primary MR). It could also be due to left ventricle disease resulting in chordal stretching, or enlargement of the mitral annulus (secondary MR). Several mechanisms often overlap in chronic MR16. A combination of several plasty techniques (valvular, chordal and annular plasties) to address all mechanisms participating in MR allowed surgical repair to eliminate significant MR in almost all patients17,18. As a result, some authors advocated that combined procedures using transcatheter plasty techniques could yield landmark surgical results19. However, transcatheter mitral plasty systems are highly complex, involving difficult learning curves10. Combined procedures are also longer (which increases the thrombotic risk) and costlier. Thus, combined transcatheter plasty procedures to treat multifactorial MR are cumbersome, which limits their adoption.
Historically, surgical repair has been widely recognised as being superior to replacement for the treatment of primary MR because it resulted in lower mortality17,20,21,22, although this idea is only supported by small observational studies subject to confounding, and with short follow-up17. However, recurrent MR is constantly more frequent after surgical repair than after replacement15,17. Furthermore, recent randomised trials comparing surgical repair and replacement for the treatment of ischaemic MR found that replacement yielded higher risk of surgery-related perioperative mortality. This could be due to the extracorporeal circulation and left ventricle adaptation to the brutal increase in afterload due to the disappearance of MR. However, repair yielded higher recurrent MR, reoperation or late mortality rates, leading the Kaplan-Meier curves of mortality to converge at two years. Indeed, replacement nearly eliminated the risk of long-term recurrent significant MR at two years: 58.8% after repair versus 3.8% after replacement23,24. One could speculate on whether these data can be extrapolated to patients with primary MR who theoretically have a more adaptable myocardium to confront the brutal increase in afterload resulting from the correction of MR. Furthermore, percutaneous mitral intervention could allow operators to avoid invasive processes such as cardiopulmonary bypass and mechanical ventilation, which could in turn improve intervention-related perioperative mortality while preserving late outcomes by yielding better MR persistence or recurrence prevention (Figure 1).
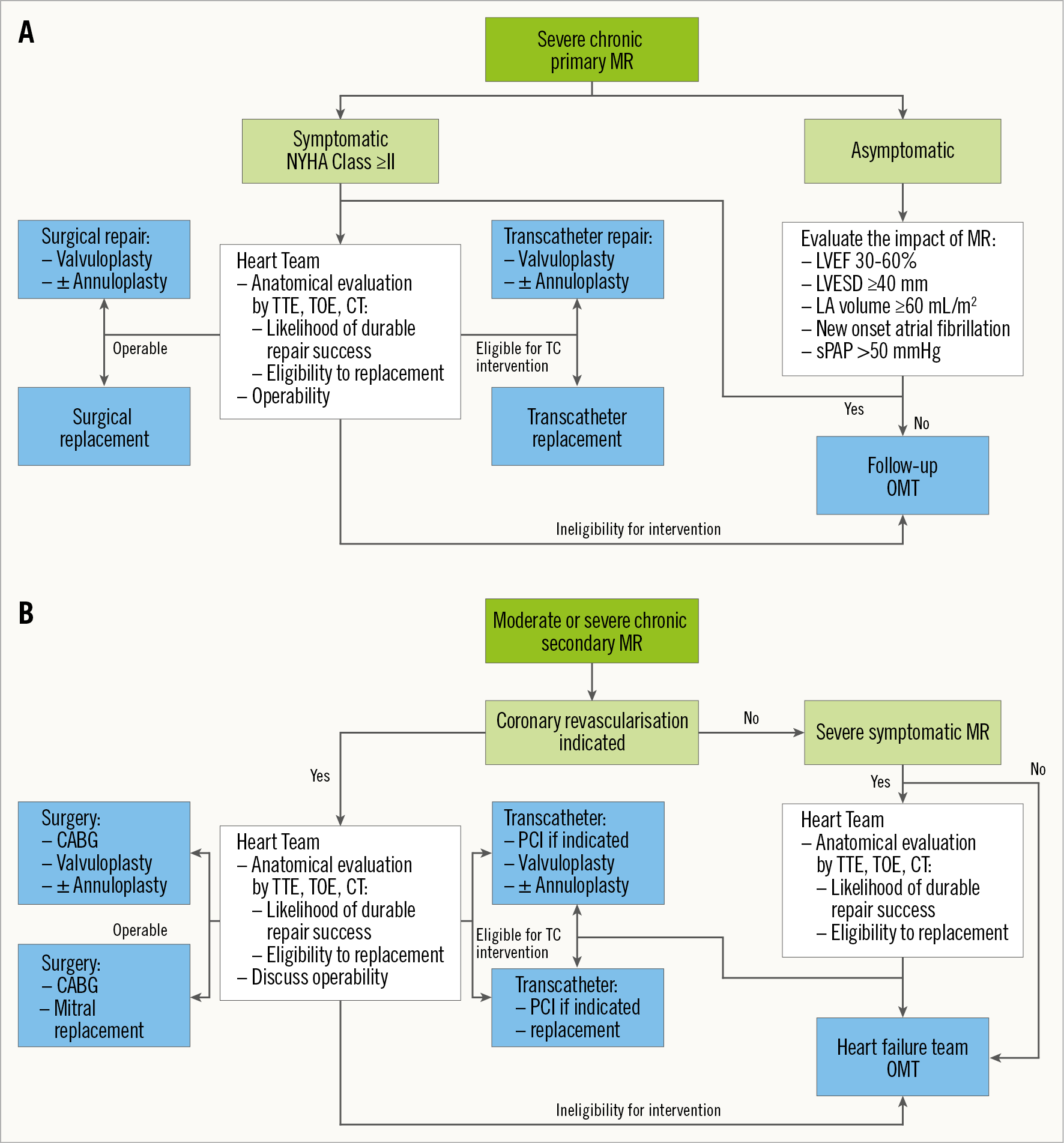
Figure 1. Proposed interventional algorithm for primary (A) and secondary (B) mitral regurgitation. CABG: coronary artery bypass graft; CT: computed tomography; LA: left atrium; LVEF: left ventricle ejection fraction; LVESD: left ventricle end-systolic diameter; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; sPAP: systolic pulmonary artery pressure; TC: transcatheter; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography
A plea for further research on TMVR
The first-in-human TMVR was performed with the CardiAQ valve (Edwards Lifesciences, Irvine, CA, USA) in 201225. Recently published observational data with the Tendyne (Abbott Vascular) and Intrepid™ (Medtronic, Minneapolis, MN, USA) devices implanted transapically in very high surgical risk patients are encouraging. Indeed, similarly to surgical replacement, transcatheter replacement yielded constant MR elimination after the intervention26,27.
Based on surgical data, TMVR is likely to yield lower rates of persistent and recurrent MR as compared to transcatheter repair. The results of the APOLLO randomised trial (NCT03242642) that compares TMVR with the Intrepid prosthesis to surgery for the treatment of primary and secondary MR could provide the first significant breakthrough in TMVR. Besides, numerous other transapical and transseptal TMVR feasibility and safety single-arm studies are also underway (NCT02722551 – RELIEF trial [CardiAQ™; Edwards Lifesciences], NCT02974881 – HighLife™ TMVR System Study [HighLife™; HighLife SAS, Paris, France], NCT02768402 – PRELUDE study [Caisson; Caisson Interventional LLC, Maple Grove, MN, USA], NCT02276547 – TIARA-I [Tiara™; Neovasc Inc., Richmond, BC, Canada]).
Indeed, if TMVR is to succeed, it will require to be feasible percutaneously through the transseptal approach. Experience with TAVI has proven that transapical was the most morbid of approaches, due to the bleeding risk and prolonged perioperative care to treat anaesthetic and surgical complications28. Nevertheless, the transition from the current 32-45 Fr transapical delivery catheters to transseptal compatible delivery systems will require engineering modifications in size, valve design and delivery methods.
Overall, in the next few years, if transseptal TMVR is proven to be feasible and safe, the long-awaited advent of transcatheter treatment of MR will finally arrive. We advocate that transcatheter replacement should provide a more effective solution than repair to treat most cases of MR and, by avoiding the excessive perioperative morbidity/mortality associated with surgical replacement, it will yield better short- and long-term clinical outcomes as well as fewer MR recurrences than repair. More research and technical improvement of TMVR device and delivery system designs are necessary.
Conflict of interet statement
T. Modine is a consultant for Boston Scientific, Medtronic, Edwards, Cephea, MicroPort, GE, and Abbott; he has received a research support grant from Edwards. B. Prendergast has received speaker fees from Edwards Lifesciences. N. Piazza declares being a consultant/proctor for HighLife, Medtronic and MicroPort, and is a consultant for Cephea. P. Overtchouk has no conflicts of interest to declare.

