Twenty years after the first human use of the MitraClip system (Abbott), mitral transcatheter edge-to-edge repair (M-TEER) has initiated a new era in the treatment of severe mitral regurgitation (MR). Following CE (European conformity) marking in 2008 and U.S. Food and Drug Administration approval for the treatment of primary (PMR) and secondary MR (SMR) in 2013 and 2019, respectively, M-TEER continues to address the unmet needs of thousands of patients who are not eligible for surgery. Several iterations have since been introduced, and the safety and efficacy of the technique have been demonstrated in several observational studies and randomised controlled trials123 with more than 200,000 patients treated mainly in Europe and the USA. In parallel, the development of the PASCAL device (Edwards Lifesciences), an alternative M-TEER system that was the first to involve independent leaflet capture, has broadened the range of therapeutic options, while showing comparable results in smaller studies45.
Nowadays, patients with growing anatomical complexity, going far beyond the initially defined EVEREST criteria6, are selected for M-TEER by increasingly experienced operators and imaging teams78. Eligibility has also been expanded to more acute clinical scenarios like cardiogenic shock9 and relevant SMR after myocardial infarction10. However, concerns of a higher procedural failure rate associated with difficult valve anatomies have emerged11, stimulating the development of more versatile systems with several implant sizes, as well as transcatheter valve replacement as an alternative treatment modality. Despite these technological evolutions, which have already translated into improved patient outcomes12, the adoption of this therapy remains limited in many parts of the world (Figure 1). The geographical disparities described for transcatheter aortic valve implantation (TAVI)13 are much more pronounced for M-TEER even among Western countries, and penetration remains largely below the usually expected rate of 1 M-TEER procedure for 3 TAVIs. While high geographical disparities exist within Europe, market estimations suggest that TAVI is performed 12 times more frequently per million inhabitants in France and 17 times more in the United Kingdom compared to M-TEER. The reasons for these observations are multiple, including insufficient disease and therapy awareness, diverging reimbursement policies, as well as high procedural complexity and costs.
The DragonFly-M system (Valgen Medtech) is a new type of M-TEER device that was first implanted in China in 202114. It has the ability to adjust leaflet tension by means of a central compressible filler and includes 4 device sizes (width 4-6 mm, length 9-12 mm). A feasibility study demonstrated encouraging results at 30 days for the treatment of severe PMR and SMR15.
In this issue of EuroIntervention, Wang et al report the results of the DRAGONFLY-DMR study, which investigated the 1-year results of the DragonFly-M system in 120 M-TEER patients at 27 centres across China16. The inclusion period extended from May 2021 to January 2022. To be eligible, patients were required to have symptomatic chronic moderate-to-severe (3+) or severe (4+) PMR, to be high risk for surgical mitral valve repair, and anatomically suitable for M-TEER with the DragonFly device, as assessed by two independent experienced operators. The primary efficacy endpoint was clinical success at 1 year, defined as freedom from mortality, reintervention for mitral valve (MV) dysfunction, and moderate-to-severe or severe MR (>2+). Safety endpoints included major adverse events (MAE; defined as procedure-related mortality, stroke, myocardial infarction, renal failure, and cardiovascular reintervention related to the procedure or device), and all-cause and cardiovascular mortality16.
The mean age at baseline was 74.9±5.7 years (49.2% female). The mean Society of Thoracic Surgeons score for MV replacement was 6.9±2.8%, and two-thirds of the patients (65.9%) were in New York Heart Association (NYHA) Functional Class ≥III at baseline. More than 70% had severe PMR (4+), and the remaining patients had MR grade 3+. The most common cause of MR was prolapse (75.8%), frequently located at the P2 (55.8%). The DragonFly device was successfully placed in all but one patient (119/120; 99.2%), in whom the implant was removed because of inadequate MR reduction. The mean number of implants was 1.5±0.6, most frequently in the A2-P2 location (71.3%), and the widest and longest device (XW0612: width 6 mm, length 12 mm) was used most frequently (60.3%). Clinical success was achieved in 87.5% of patients at 1 year (95% confidence interval [CI]: 80.1-92.3%), and MR severity was durably improved to moderate or less (≤2+) in 92.0% (p<0.001). There was also a significant reduction in postoperative left atrial pressure (−4.8 mmHg on average; p<0.001), with significant left ventricular reverse remodelling at 1 year. The mean MV gradient increased by <1 mmHg and remained stable throughout 1 year. The MAE rate was 9.2% (including 2.6% strokes and 2.6% cardiovascular reintervention related to the procedure or device), all-cause mortality was 5% (all deaths occurred before 6-month follow-up), and cardiovascular mortality was 4.2%. Heart failure hospitalisation (HFH) occurred in 3.7% of patients at 1 year. For the sake of comparison, the 1-year HFH rate in the EXPAND G4 Study was 10.4% for PMR3, while it was 8.1% in the PASCAL group and 3.3% in the MitraClip group in the CLASP IID Trial5. In addition, at 1 year, NYHA Class and quality of life improved significantly by a mean of 31.2 points according to the Kansas City Cardiomyopathy Questionnaire score, which corresponds to a very large change17.
Although the described results are promising for a first-generation device used in a country with low M-TEER penetration, some key aspects of the study and results require further attention. First, most patients with complex MV anatomies (such as MV orifice area <3.5 cm2, posterior leaflet length <8 mm, prolapse/flail gap >10 mm, Barlow’s disease or multisegmental prolapse, presence of multiple jets, or presence of severe calcification of the annulus or in the grasping area) were excluded. Although strict eligibility criteria are expected in a pilot study, the possibility of treating a complex mitral anatomy with the DragonFly device still needs to be demonstrated. Second, the proportion of MR ≤mild (≤1+) was 85.6%, 64.3%, 62.6%, and 69.7% respectively, at discharge, 30-day, 6-month and 1-year follow-ups. Compared to recent studies performed in comparable populations treated with the last generation of the MitraClip (90% reduction to MR ≤1+ in the EXPAND G4 Study at 30 days) or PASCAL devices (86% reduction to MR ≤1+ in the CLASP IID Trial at 30 days), these rates seem relatively low, which could be either device or operator dependent. Importantly, in patients with PMR, achieving residual MR ≤1+ is of prognostic relevance and should be the treatment target18.
Therefore, when interpreting these results, one has to consider that the study was conducted exclusively in China, where the procedure is relatively new, with most sites having no or little previous M-TEER experience. Furthermore, due to the global coronavirus pandemic, proctoring had to be performed remotely. Still, procedural duration decreased during the study period, indicating an encouraging learning curve. Although additional efforts are necessary to optimise procedural MR reduction using the DragonFly system, and continuous monitoring of the safety and efficacy of the procedure is warranted to reach current benchmarks, initiatives that improve access to transcatheter treatment for high-risk patients without therapeutic alternatives should be encouraged and accompanied by experts with appropriate experience. However, technical solutions adapted to local specificities will not suffice to enable M-TEER to conquer the globe: further efforts regarding disease and therapy awareness are needed.
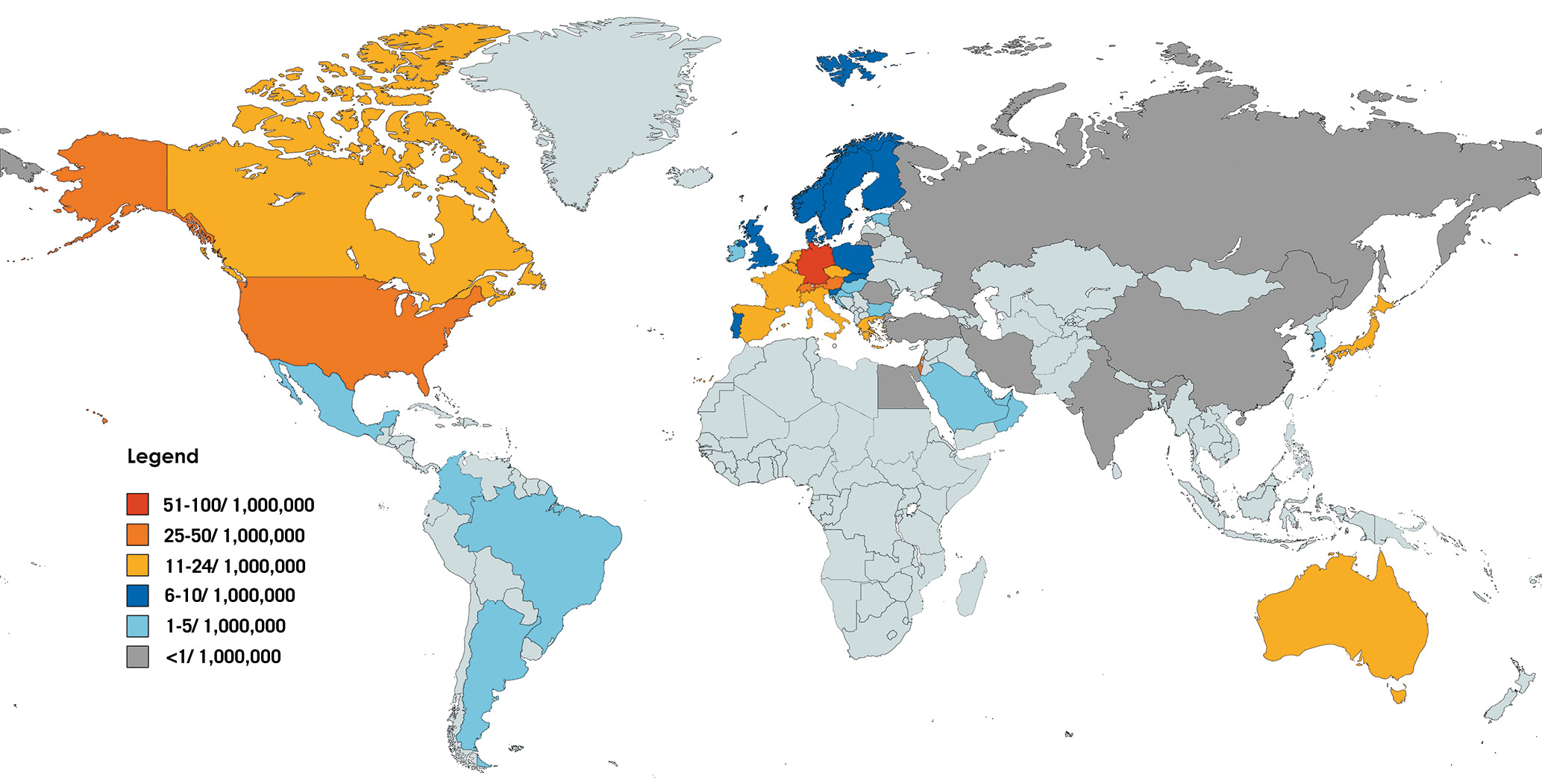
Figure 1. Geographical dispersion of mitral edge-to-edge procedures. M-TEER procedures per 1,000,000 inhabitants during the year 2022 (Source: data on file, Abbott). M-TEER: mitral transcatheter edge-to-edge repair
Conflict of interest statement
F. Praz was compensated for travel expenses by Abbott, Edwards Lifesciences, Polares Medical, Medira, and Siemens Healthineers. D. Samim received funding for an online course from Edwards Lifesciences.

