Abstract
In the last year transcatheter mitral valve implantation (TMVI) has seen a major jump in development. This technique offers the potential to treat a great number of elderly and/or high-risk patients with severe mitral regurgitation (MR). Such patients are declined surgical intervention either because the institutional Heart Team considers the risk of intervention to exceed the potential benefit, or because the patients and their families believe the morbidity of mitral surgery to be excessive. The advent of a less invasive transcatheter treatment could, therefore, potentially appeal to both clinicians and patients alike. In this overview paper, we describe briefly these recent developments in TVMI technologies as an introduction to the dedicated TVMI technical device parade later in this supplement.
Finally! The past year has seen transcatheter mitral valve implantation (TMVI) begin in earnest. As the prevalence of mitral valve disease is almost three times that of aortic valve disease1, this technique offers the potential to treat a great number of elderly and/or high-risk patients with severe mitral regurgitation (MR). Indeed, the Euro Heart Survey suggested that half of all patients hospitalised with symptomatic severe MR do not undergo potentially curative surgical repair/replacement due to advanced age, comorbid illnesses, and left ventricular dysfunction2,3. More specifically, as few as 16% of patients with severe symptomatic functional MR and 53% of those with degenerative MR actually undergo surgery4. Furthermore, population-based data suggest that in the USA only 2% of eligible patients (MR ≥grade 3) undergo mitral valve surgery5-7. Such patients are declined surgical intervention either because the institutional Heart Team considers the risk of intervention to exceed the potential benefit8, or because the patients and their families believe the morbidity of mitral surgery to be excessive. The advent of a less invasive transcatheter treatment could, therefore, potentially appeal to both clinicians and patients alike.
Of course, there remains a great deal to learn about which patients could benefit from TMVI. Dr Elliot C. Cutler performed the first surgical mitral valve repair in 19239, yet the mode of repair/replacement and the timing of the intervention still remain topics of some debate10,11. As with transcatheter aortic valve implantation (TAVI), patient selection is determined by anatomical and clinical criteria. Both involve complex decision matrices which require much clarification. Anatomically, TMVI is a veritable minefield: a large, non-circular, saddle-shaped, highly dynamic, non-calcified annulus without the ability for radial anchoring which is tethered to a complex, highly individualised, subvalvular apparatus, and intimately related to the left ventricular outflow tract (LVOT), the coronary sinus, and the left circumflex coronary artery. Detailed multislice computed tomography analysis is imperative for patient selection and preoperative procedural planning (Figure 1)12. Clinically, while patient selection continues to evolve, there remain significant questions regarding the implications of large delivery sheath insertion in the left ventricle and the restoration of mitral valve competency in patients with very poor left ventricular function.
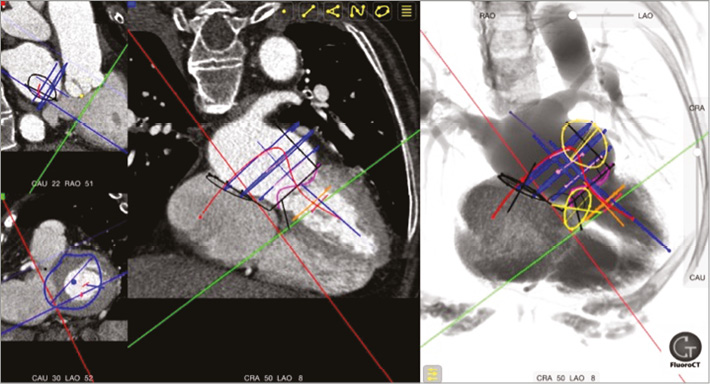
Figure 1. Mitral valve CT analysis. Multislice computed tomography of the mitral valvular complex using the FluoroCT App.
On June 12th, 2012, Lars Søndergaard and the Heart Team at Rigshospitalet in Copenhagen, Denmark, performed the first TMVI on an inoperable 86-year-old patient13. Using the first-generation CardiAQ valve system (CardiAQ Valve Technologies, Inc., Irvine, CA, USA), a successful transfemoral transseptal implantation was achieved with stable valve position and haemodynamics14. Although the patient succumbed to multi-organ failure on postoperative day three, TMVI feasibility had been demonstrated.
To date, five transcatheter mitral valve systems have been implanted in humans: CardiAQ valve system (CardiAQ Valve Technologies, Inc.); Tiara™ valve (Neovasc Inc., Richmond, Canada); FORTIS valve (Edwards Lifesciences, Irvine, CA, USA); Tendyne valve (Tendyne Inc., Roseville, MN, USA); and Twelve valve (Twelve, Inc., Redwood City, CA, USA). These devices share common features: nitinol self-expanding frames, trileaflet valves, bovine pericardial leaflets (Tendyne is porcine), fabric sealing skirt (CardiAQ is pericardial), and transapical delivery (CardiAQ also transseptal). Each of these systems, and those in preclinical development (Medtronic Mitral15 [Medtronic, Minneapolis MN, USA]; HighLife [HighLife Inc., Paris, France]), offer innovative design solutions to overcome the challenging anatomy of the mitral valve complex (Figure 2). TMVI systems must be flexible to deal with the complex and variable anatomy, provide large effective orifice areas, and deal with high transvalvular gradients (Figure 3). They must anchor without reliance on radial force (axial sealing), accommodate significant dislodgement forces, and avoid LVOT obstruction. Given these obstacles, additional areas of concern include stent fatigue and fracture, valve thrombosis, embolisation, leaflet durability, and paravalvular leak with resultant haemolysis.
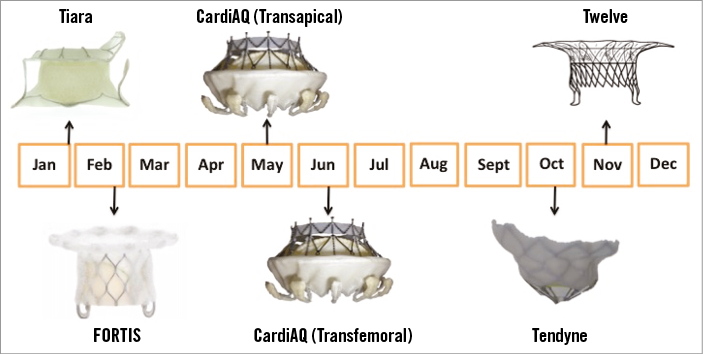
Figure 2. Transcatheter mitral valve development timeline. Tiara (Neovasc Inc.); FORTIS (Edwards Lifesciences); CardiAQ (CardiAQ Valve Technologies, Inc.); Tendyne (Tendyne Inc.); Twelve (Twelve, Inc.).
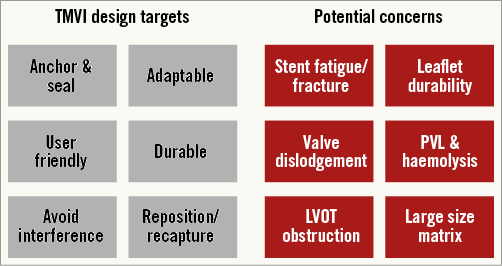
Figure 3. TMVI design targets and potential concerns. LVOT: left ventricular outflow tract; PVL: paravalvular leak
On January 30th, 2014, Drs Anson Cheung and John Webb successfully implanted the first-in-human Tiara valve (Neovasc Inc.)16. The valve frame is asymmetric to fit the D-shaped mitral annulus. The 32 Fr transapical delivery catheter allows prosthesis recapture and repositionability, and the system anchors and seals using an atrial skirt and ventricular anchoring arms17. To date, four patients have been treated with successful device position and function in all cases. All patients were alive at 30 days.
In February and March 2014, Drs Martyn Thomas and Vinnie Bapat treated the first human patients with the FORTIS transcatheter valve (Edwards Lifesciences)18. The system is delivered via a 42 Fr sheathless delivery catheter, and anchors and seals using an atrial flange and symmetric paddles that clip the mitral leaflets on the ventricular side. Among 12 reported cases, successful delivery and valve function occurred in 11 cases, and 30-day mortality occurred in 25%. In May 2015, Edwards Lifesciences announced suspension of the FORTIS programme to investigate more thoroughly the presence of valve thrombosis.
On May 13th, 2015, almost two years after performing the first-in-human TMVI with the first-generation CardiAQ valve, Lars Søndergaard and the Rigshospitalet Heart Team implanted the second-generation CardiAQ implant (CardiAQ Valve Technologies, Inc.), using a transapical delivery system. A month later, Francesco Romeo and Gian Paolo Ussia performed the first transfemoral transseptal implant of the second-generation device in Tor Vergata Hospital in Rome, Italy19. This system achieves sealing and anchoring using both left ventricular and left atrial anchors. Among eight treated patients, successful valve delivery and function was reported in seven cases. The 30-day mortality was 50%.
October 2014 witnessed the first human implants of the Tendyne valve (Tendyne Inc.) by Mr Neil Moat and the Heart Team at the Royal Brompton, London. The valve consists of a D-shaped frame that houses porcine pericardial leaflets. The prosthesis can be repositioned and is retrievable, and achieves sealing and anchoring by an atrial skirt and a left ventricular apical tether. Successful prosthesis delivery and function was achieved in all 12 patients treated. There were no 30-day deaths20.
Therefore, as PCR London Valves moves east (temporarily) to Berlin from its spiritual home in London, another important transition is evident. Transcatheter heart valve technology has moved south from the aortic to the mitral valve, and TMVI has emerged as a clinical reality. Much work is required to iterate these devices and to optimise patient selection. This journey starts at PCR London Valves (Berlin) 2015.
Conflict of interest statement
D. Mylotte has no conflicts of interest to declare. N. Piazza is a member of the Scientific Advisory Board of Medtronic and a consultant and equity shareholder with HighLife.

