
Abstract
Both transcatheter mitral valve repair and replacement have been introduced in patients with severe mitral regurgitation (MR). However, while transcatheter mitral valve repair is rapidly evolving, transcatheter mitral valve implantation (TMVI) has had a slow development path. One of the main reasons for this is the challenge to find good candidates for this therapy. Although scarce data exist for patient inclusion and/or exclusion criteria and patient screening, the current rejection rate for TMVI is reported to be around 60%. The rejection could be due to: 1) the restriction of the technique to patients at high and extreme surgical risk, as well as the exclusion of patients in whom intervention will be futile, 2) the complexity of the procedure and the anatomical constraints which are high in patients with native valve disease and in those with severe annular calcification, and 3) low procedural safety with left ventricular outflow tract obstruction as the most feared complication. In the future, new transcatheter heart valve platforms and designs are expected to be available which will reduce the periprocedural complications. Furthermore, evaluation of the mitral valve anatomy before and during the procedure may be more accurate, and more evidence on the best clinical practice is expected, including a comparison of TMVI with transcatheter mitral valve repair and surgical intervention, as well as the long-term follow-up. This will facilitate determining the subset of patients who may potentially benefit from this technology.
Abbreviations
LV: left ventricle
LVOT: left ventricular outflow tract
MAC: mitral annulus calcification
MR: mitral regurgitation
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
TMVI: transcatheter mitral valve implantation
ViMAC: valve-in-MAC
ViR: valve-in-ring
ViV: valve-in-valve
Introduction
Following the success of transcatheter aortic valve implantation (TAVI), there has been great interest in transcatheter mitral valve interventions to treat severe mitral regurgitation (MR)1. The rationale for an interventional treatment in MR is based on several arguments: the high frequency of the disease, poor clinical prognosis if severe MR is left untreated, the high-risk profile for surgery in the ageing population, and frequent denial of surgical intervention by practising physicians2.
Transcatheter mitral valve repair is rapidly developing but, although a variety of transcatheter heart valve (THV) systems has been proposed, transcatheter mitral valve implantation (TMVI) has had a slow development path3-8.
The screening failure rate at this stage seems to be high. This review aims to summarise the exclusion criteria for TMVI and to elaborate on what the future could be with regard to patient selection and, as a consequence, the use of these devices. It will also address the specificities of patients with native valve disease, severe mitral annular calcification (MAC), and failure of surgical mitral valve interventions.
General comments
The general observation is that there is a high rejection rate of candidates for TMVI. Unfortunately, only scarce data exist for patient screening and inclusion and/or exclusion criteria. It may also be assumed that many patients were rejected by physicians before being formally screened in study protocols. In addition, it needs to be kept in mind that these reasons may be different for each mitral THV system.
The slow development of TMVI could be due to several factors: the lack of candidates for the technique due to the epidemiology of the disease, the complexity of the procedure, the lack of efficacy, the low procedural safety, anatomical constraints, and the high availability of transcatheter mitral repair technologies, which have been associated with a high safety profile even in high-risk patients. In terms of the epidemiology of MR, it should be stated that the data are few and suffer from serious limitations, especially with regard to secondary MR1,9. Thus, the expected number of patients who are potential candidates for a transcatheter mitral valve intervention may be lower than expected.
The current experience with TMVI is limited to first-in-man studies and case series in patients with native valve disease and small registries in patients with MAC or failure of surgical mitral valves and rings. These studies were performed in very high-risk patients, and in patients with native valve disease mostly for secondary MR4. The efficacy seems satisfactory in terms of haemodynamics and reduction of MR. The procedural safety in the initial series of TMVI in patients with native valves or MAC was low but has improved in the most recent reports - partly due to a more restrictive patient selection with regard to clinical and anatomical features.
CLINICAL FACTORS
TMVI is currently only indicated for severe MR. However, this statement should take into account the current debate on the grading of secondary MR10,11. The presence of severe symptoms despite optimal medical management is a prerequisite for TMVI, as is the case in most patients for surgical valve replacement. At this stage of development, the use of TMVI is restricted to high-risk or inoperable patients as defined by the Heart Team. As for TAVI, when the benefit of TMVI is unlikely and the risk is high, patients should be excluded to avoid futility:
– The extracardiac reasons include comorbidities similar to those in TAVI. However, the patients with mitral valve disease are usually younger than those with aortic stenosis and, therefore, usually have less comorbidity.
– The cardiac reasons are those for which the efficacy of any intervention on the mitral valve in patients with MR and low left ventricular ejection fraction is debatable. Patients with severe tricuspid regurgitation and right ventricular dysfunction are unlikely to derive a significant benefit from the treatment of MR in isolation. The same holds true in patients with severe pulmonary hypertension.
ANATOMICAL FACTORS
– In patients with native valve disease and also, to a lesser degree, in those with MAC, the complexity of the mitral valve apparatus and the heterogeneity of the disease are important limitations due to the constraints of TMVI.
– The left ventricular outflow tract (LVOT) is by definition the part of the LV located between the anterior mitral leaflet and the ventricular septum and thereby closely related to the mitral valve apparatus. The LVOT may be obstructed after implantation of the mitral THV. Several factors increase the risk of this complication: the presence of a small LVOT and left ventricle, and a sigmoid septum, an acute aorto-mitral angulation, a calcified or elongated anterior mitral leaflet, or a calcified anterior subvalvular apparatus12 (Figure 1). In addition, a dynamic component might have an impact on the risk of LVOT obstruction. Even patients with a favourable anatomy could have a certain degree of LVOT obstruction if this aspect is not taken into consideration. The risk of this major complication must be carefully evaluated by multimodality imaging before TMVI (Figure 2)13.
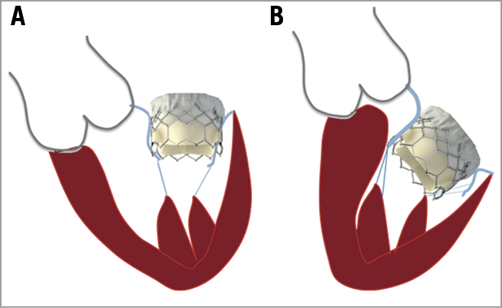
Figure 1. Factors associated with left ventricular outflow tract obstruction after transcatheter mitral valve implantation. A) Low risk of left ventricular outflow tract obstruction. B) High-risk anatomy due to a small left ventricle cavity, an acute aorto-mitral angulation, a sigmoid ventricular septum. Note the transcatheter heart valve protruding into the left ventricle.
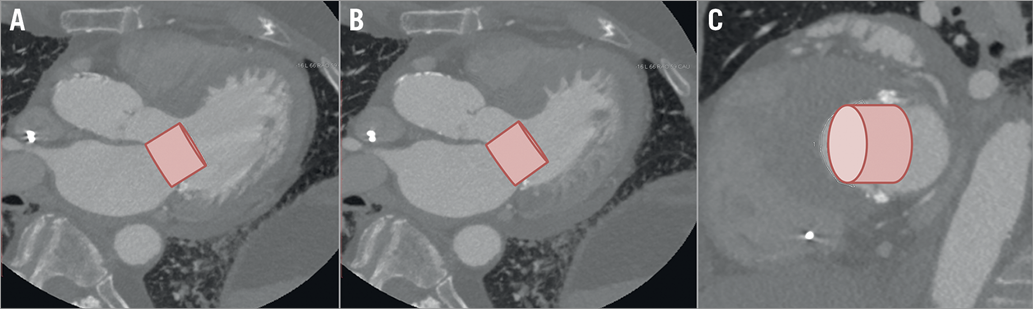
Figure 2. Computed tomography reconstructions with a simulated SAPIEN 3 THV in a candidate for TMVI showing a high risk for LVOT obstruction. A) Three-chamber view, in diastole, B) three-chamber view, in systole. Note the proximity of the simulated THV to the septum. C) Short-axis view in systole showing cross-section of the LVOT.
– The mitral valve is the most difficult valve to access for transcatheter valve therapies. The mitral apparatus can be reached anterogradely by either the transseptal or the transatrial route, or retrogradely by either the transapical or the transaortic route. The most common approaches utilised in current transcatheter mitral valve therapies are the transapical and transseptal routes, followed in a limited number of cases by the transatrial approach. Compared to the aortic annulus, the mitral annulus is larger, requiring larger mitral THVs. As a consequence, the profile of the delivery systems is currently 30-40 Fr for the new devices used in patients with native valve disease. These large core systems may be difficult to deploy via a transseptal route; the main approach has therefore been transapical access. Transapical access to the mitral valve provides several advantages for the design of the delivery system and for the implantation technique since both are easier when working with straight systems rather than in a 180° angle as required for the transseptal approach. Indeed, coaxiality, which is crucial for successful TMVI, is more easily achieved using the transapical approach. However, crossing the apex of the left ventricle with 30-34 Fr delivery systems may be associated with a high risk of complications and poor outcomes, in particular in patients with left ventricular dysfunction which is frequently observed in patients with functional mitral regurgitation. On the other hand, less invasive transseptal procedures are desirable in order to lower morbidity and mortality rates in the high-risk TMVI candidates who frequently have low LV ejection fraction12-14.
TMVI in patients with native mitral valve disease
The experience of TMVI in patients with native mitral valve disease is still limited to a few hundred patients4-8. In this subgroup there are several challenges:
– The mitral annulus is not a flat and circular structure, but rather can be described as D-formed and saddle-shaped. The annulus is dynamic during the cardiac cycle and becomes deformed as the disease progresses. Thus, the size of the mitral annulus can vary by up to 30% during the cardiac cycle. The shape of the annulus also changes during the cardiac cycle with a more circular shape in late diastole and a more elliptical shape during systole. Thus, anchoring of a THV in the native, non-calcified mitral annulus solely by radial force may be challenging. The design of a mitral THV needs to include not only proper device sizing, but also a stent frame that is robust enough to resist the radial forces exerted throughout the cardiac cycle and avoid stent fracture while not being too rigid with the risk of impingement of surrounding structures. A high radial force of the THV will imply a risk of compression to adjacent structures such as the LVOT, coronary sinus, and circumflex artery. As a consequence, most mitral THVs also use other kinds of anchoring mechanism such as the mitral leaflets and a tether anchored at the left ventricle apex. Furthermore, the mitral apparatus also includes chordae and papillary muscles, which are important to preserve during valve replacement. Figure 3 shows the main THV used for the treatment of native mitral disease.
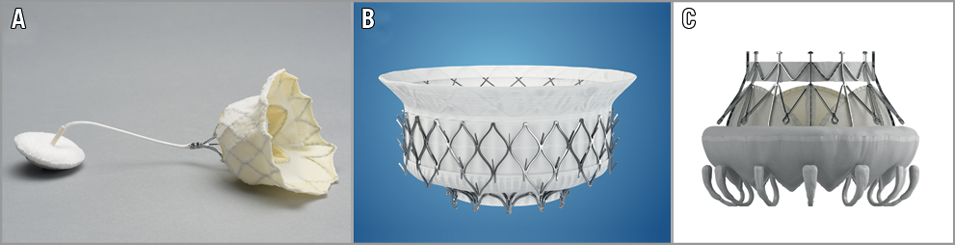
Figure 3. Transcatheter heart valves used for the treatment of native mitral valve disease. A) Tendyne Mitral Valve System (courtesy of Tendyne Holdings, LLC, a subsidiary of Abbott Vascular, Roseville, MN, USA). B) Intrepid TMVR System (courtesy of Medtronic, Inc., Redwood City, CA, USA). C) CardiAQ - Edwards Transcatheter Mitral Valve (courtesy of Edwards Lifesciences LLC, Irvine, CA, USA).
– A careful patient selection is probably one of the main factors which led to better safety in the most recent trials in comparison with the earlier ones6,8,9. Details concerning the exclusion criteria have only been reported in two studies7,8 which show the high screening failure as being 70% and 60%, respectively (Table 1).
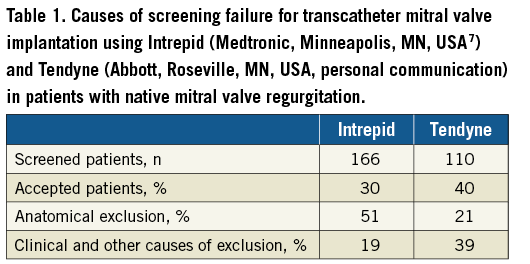
The most comprehensive description is given in the Intrepid Global Pilot Study (Medtronic, Minneapolis, MN, USA) where a total of 166 patients were screened for possible study participation and only 50 (30%) patients were enrolled7. The most common reason for exclusion was the lack of anatomical suitability, which was evident in 84 (50%) patients, comprising:
– Concern for LVOT obstruction (22%)
This potential complication is worrisome in this patient subgroup when the prosthesis protrudes in the LV.
– Too large a native mitral valve (20%)
In this patient group, the diameter of the mitral annulus may be approximately double that of the aortic annulus. This translates into a four times larger circumference of the mitral annulus and therefore the need for large-size mitral THV stent frames and delivery systems, which are less flexible than the current TAVI delivery systems. Currently, most mitral THV systems have a limited size range and are therefore not suitable for very large or small mitral annuli.
– Too small a native valve in 8% of patients
This figure may increase if TMVI is used in patients with rheumatic valvular heart disease.
Of the 82 patients with suitable anatomy, 32 patients were excluded. Reasons for exclusion were:
– Poor left ventricular function (4%)
Currently, most trials of TMVI systems exclude patients with LV ejection function <30%. As stated earlier, most TMVI systems have been introduced transapically. Since approximately 75% of TMVI patients have functional MR, transapical access may be a major limitation for the expansion of the therapy due to the risk of post-procedural LV apical “stunning”, which may not be well tolerated.
– Low surgical risk (3%)
Due to the relatively high mortality and morbidity of TMVI compared to surgery and transcatheter mitral valve repair, the therapy is currently restricted to patients at high or prohibitive surgical risk.
– Presence of a mechanical aortic valve prosthesis (3%)
Some mitral THVs use anchors at the ventricular site of the mitral leaflets for fixation, which may potentially interfere with the tilting discs of mechanical aortic valve prostheses.
– Severe mitral annular calcification (1%)
Calcification of the mitral annulus may both compromise the anchoring of the mitral THV and increase the risk of paravalvular leakage.
– Other factors (10%)
These include mitral regurgitation less than severe, other mitral treatment, futility, patient refusal, patient no longer symptomatic, baseline systolic anterior motion, and severe pulmonary hypertension.
TMVI in patients with severe calcification of the mitral annulus
Surgical mitral valve replacement in the presence of MAC is associated with a high risk of life-threatening complications such as mitral annulus rupture, rupture of the free wall of the left ventricle and damage to the circumflex artery. These patients, mostly considered inoperable, may benefit from percutaneous therapy.
TMVI has recently been used for the treatment of severe mitral disease in patients with a severe MAC (Figure 4A). The SAPIEN (Edwards Lifesciences, Irvine, CA, USA) family of THVs is mainly used in these patients, although some dedicated TMVI devices such as the Tendyne (Abbott, Roseville, MN, USA) and the Tiara™ (Neovasc Inc., Richmond, BC, Canada) THVs have also been implanted in patients with a MAC with promising results. Nonetheless, only about 10% of these patients are good candidates for TMVI12. Clinical and anatomical factors limit the use of this therapy in this group of patients.
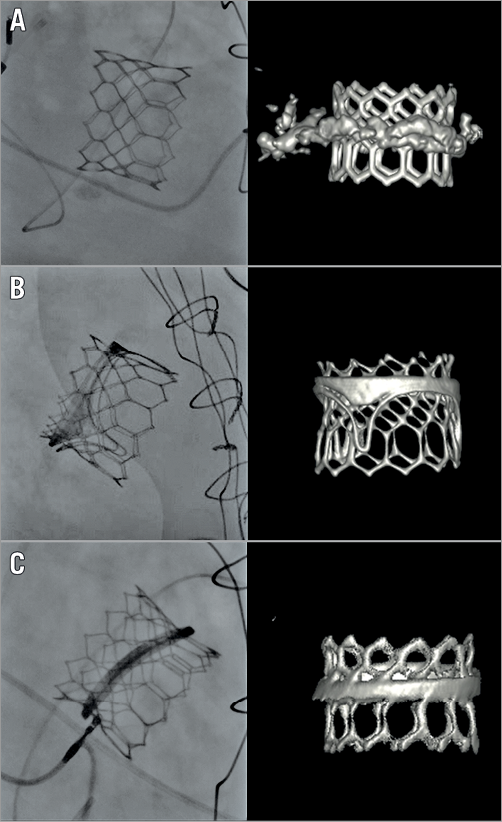
Figure 4. Fluoroscopic and volume-rendering computed tomography images showing the results of a valve-in-MAC, valve-in-valve, and valve-in-ring. A) Valve-in-MAC. B) Valve-in-valve. C) Valve-in-ring. MAC: mitral annular calcification
CLINICAL FACTORS
MAC is associated with the presence of cardiovascular risk factors, e.g., atrial fibrillation, coronary artery disease, conduction abnormalities, stroke and other heart valve diseases such as aortic stenosis15,16. Thus, it may be difficult to identify precisely which symptoms are associated with the mitral valve disease and frequently these patients are not referred for intervention or are not referred before it is too late. Likewise, initial case series of TMVI in patients with a MAC included patients at very high risk for surgery with a mean STS score >10%. This resulted in a high rate of periprocedural mortality (~30%)17 from non-cardiac causes in more than half of them.
ANATOMICAL FACTORS
The main limiting anatomical factors for TMVI include:
1. Anchoring and sealing of the mitral THV
The mitral annulus is larger than the aortic annulus, and therefore transcatheter devices available and used in clinical practice up until now may be too small to be used in the mitral position. Furthermore, a more important degree of oversizing is necessary to compensate for the differences of pressure between the left ventricle and atrium. The extension and location of calcifications might be a limiting factor for TMVI. Calcifications are frequently non-circumferential and exclusively located in the posterior part of the mitral annulus. In the absence of a mechanical aortic prosthesis or calcification of the mitral trigones, the risk of valve embolisation is too high in these patients and TMVI is contraindicated12. Also, TMVI using a SAPIEN THV should be avoided in patients with rheumatic mitral stenosis where the most severe obstruction is not at the level of the annulus but deeper in the left ventricle.
2. Risk of LVOT obstruction
This is one of the main causes of denial of TMVI in patients with a MAC and the most feared complication of these procedures, being associated with a mortality >80%17. Patients with a MAC are frequently women with hypertrophic ventricles and a small LVOT12. As stated before, the size of the LVOT is one of the more important factors associated with the risk of LVOT obstruction. Therefore, only a small proportion of these patients is finally eligible for this intervention. Although the exact criteria for LVOT obstruction in patients with a MAC remain to be determined, it is possible to identify accurately those patients who are at low risk of this complication and who will benefit from TMVI using multimodality imaging13.
TMVI after cardiac surgery: valve-in-valve (ViV) and valve-in-ring (ViR) procedures
Transcatheter aortic valve-in-valve (ViV) implantation is nowadays routinely used for the treatment of patients with degenerated surgical bioprosthetic aortic valves, being endorsed with a Class IIa recommendation in the last European guidelines10. In recent years, this technology has been extended to the treatment of patients with failing surgical mitral bioprostheses (ViV) and rings (ViR)18 (Figure 4B, Figure 4C). However, the expansion of this therapy to the mitral valve is slow and the procedure is not yet recommended in the guidelines. Clinical, anatomical and specific prosthesis and ring-related factors might explain the slower growth compared to aortic procedures. While the rate of screening failure in patients with a failing bioprosthesis is relatively low, in our experience only 70% of patients with a failing ring referred for TMVI are proper candidates.
CLINICAL FACTORS
Patients with mitral valve disease are younger than those with aortic stenosis. Contemporaneous series have shown a reduced risk of redo surgery with a perioperative mortality of 7-12%, and even down to 5% in patients with non-urgent procedures19. TMVI procedures are technically demanding, may be associated with a higher risk of complications if performed by inexperienced centres and, up until now, are mostly performed using a transapical approach, which has been associated with an increased risk of complications. Thus, TMVI is today restricted to patients at high risk for cardiac surgery.
ANATOMICAL FACTORS
The main anatomical limitation to ViR and to a lesser extent ViV procedures is the risk of LVOT obstruction. The absence of anterior leaflet in patients with a surgical mitral bioprosthesis results in a low risk of LVOT obstruction. However, this complication might occur as a result of the covering of the struts of the transcatheter prosthesis by the leaflets of the surgical valve or if the bioprosthesis is exaggeratedly oriented towards the interventricular septum, which is observed after surgery in acute endocarditis with severe lesions of the mitral annulus.
On the other hand, the risk of LVOT obstruction during TMVI is relatively high for ViR, with about 8% reported in initial case series18, and even higher in patients with a repaired and elongated anterior leaflet12.
“BIOPROSTHESIS/RING” FACTORS
Balloon-expandable THVs (Edwards SAPIEN XT or SAPIEN 3) are used in most cases. The size of the surgical prosthesis/ring is one of the main limiting factors for TMVI in these patients. Patients with small mitral bioprosthetic valves or rings precluding the implantation of a 26 mm THV may not derive a clinical benefit from TMVI due to high residual transmitral gradients, except for those patients with a very low body surface index. Although recent case reports have suggested the feasibility of fracturing the surgical prosthesis using non-compliant balloons to reduce the risk of patient-prosthesis mismatch, these results are very preliminary and patients at high risk for high residual gradients are frequently referred for cardiac redo surgery20.
Likewise, in patients with very large mitral bioprosthetic valves or rings (>33 mm), reoperation might be preferable to TMVI, given the risk of valve embolisation when TMVI is performed using the currently available THVs.
Patients with rigid rings, in particular those with an oval shape, and those with incomplete rings are not good candidates for TMVI due to the high risk of residual gradients, paravalvular leak, and LVOT obstruction. Also, in patients with radiolucent rings or mitral bioprosthetic valves, the risk of malposition of the THV is much higher; therefore, these patients are more frequently referred for cardiac reoperation instead of TMVI12.
Other findings such as the presence of severe paravalvular leaks or partial disinsertion of the mitral bioprosthetic valve or ring might result in a lack of clinical benefit and poor haemodynamic results of TMVI. Thus, these paravalvular leaks should be carefully looked for before ViV and ViR are planned, given that these patients might benefit from cardiac reoperation or combined transcatheter procedures with both device closure of the paravalvular leak and ViV or ViR.
Future
We are currently at an early stage of development of TMVI. Future improvement is expected in different domains: technology will presumably evolve (i.e., retrievable and repositionable devices), larger devices will be available, lower profiles of the delivery systems will allow a transseptal approach as default access, new platforms and different valve designs may be available for more accurate anchoring and decrease of the risk of LVOT obstruction21, periprocedural complications will be reduced with increasing experience and improved device and delivery system iterations, evaluation of the valve anatomy before and during the procedure may be more accurate due to multimodality imaging allowing prediction of LVOT obstruction and individualisation of the choice of the THV, and finally evidence from registries and RCTs with long-term follow-up will be available. Thus, the indications and contraindications for TMVI may change in the future (Table 2) and several current anatomical limitations will be addressed.
As usually happens, when devices become commercially available, i.e., when a greater experience is available, there will be fewer screening failures. It may be foreseen that the percentage of patients excluded for anatomical reasons, such as LVOT obstruction, will be less in the future. The use of preventive therapies such as alcohol septal ablation and the development of computer simulation systems to predict the risk of LVOT obstruction more accurately will allow the inclusion of patients who are excluded today.
– Most patients currently undergoing TMVI have secondary MR. The results from the recently completed RCTs in secondary MR such as MITRA-FR22 and COAPT will be informative, as regards the efficacy of the intervention in this indication, even if they concern transcatheter repair and not replacement. Furthermore, other trials will be conducted using TMVI.
– In parallel, it is expected that experience will be gained in high-risk patients with primary MR even if they are less numerous. The majority of candidates for TMVR will have enlarged left ventricles and atria because of the advanced stage of the disease. The patients with severe extracardiac comorbidities, who present with degenerative disease and recent rupture of chordae, will be excluded from TMVR because of the size of the left ventricle and atrium.
– TMVI will be used in lower-risk patients if new evidence supports it.
– Today, no data exist as to whether or not the patients selected for TMVI were considered suboptimal candidates for transcatheter repair. The choice of the most appropriate transcatheter technique will depend on the comparative results of repair techniques, isolated or in combination vs. TMVI. It is likely that both techniques will be complementary as is the case in surgery.
– TMVI is doable after the failure of surgical or transcatheter mitral annuloplasty, but not after MitraClip implantation. This may change after iteration of the MitraClip technique.
– Transcatheter treatment of multiple valve disease is in its infancy but is rapidly developing. When MR is present, severe tricuspid regurgitation is frequently present requiring transcatheter tricuspid intervention23.
– TMVI may be considered in selected patients with MAC or surgical annuloplasty with the risk of LVOT obstruction, using preventive measures such as transcatheter laceration of the anterior leaflet (LAMPOON technique), preventive septal ablation, the use of retrievable devices or a hybrid approach allowing anterior leaflet resection24 and a PM implantation to induce dyssynchrony. However, current experience in these procedures is limited and more data are necessary before these therapies can be recommended. Finally, several case reports have shown a successful treatment of LVOT obstruction with bail-out alcohol septal ablation and the implantation of a stent in the LVOT25.
Conclusions
TMVI is “a young therapy” and many issues remain to be solved. Besides the progress in technology, imaging, and procedural management, it will be of the greatest importance to collect comprehensive information on patient selection in future studies in order to determine better the subset of patients who may potentially benefit from this technology.
Conflict of interest statement
A. Vahanian has received speaker fees from Edwards Lifesciences and is a consultant for Abbott Vascular and Mitraltech. L. Søndergaard has received consultant fees and institutional research grants from Abbott, Edwards Lifesciences, and Medtronic. M. Urena has no conflicts of interest to declare.

