
Abstract
Aims: The study sought to assess outcomes of transcatheter mitral valve-in-valve implantation (TMVIV) for degenerated bioprostheses and transcatheter mitral valve-in-ring implantation (TMVIR) for failed annuloplasty rings according to access route and the Mitral Valve Academic Research Consortium (MVARC) criteria.
Methods and results: Twenty-four patients (72±13 years; eight men [33%]) underwent TMVIV (n=14) or TMVIR (n=10) for mitral regurgitation (MR; n=17) or stenosis (n=7) using balloon-expandable bioprostheses. Transapical (TA) access was chosen in 13, and transseptal (TS) access in 11 patients. MVARC technical success, device success and procedural success were 95.8%, 41.7% and 33.3%, respectively, with no differences between access routes. Cardiac output (CO) increased significantly by 1.1±0.8 l/min in TS patients, but not in TA patients (ΔCO=0.0±0.5 l/min; p=0.0051). Overall three-year survival was estimated at 57.6% (95% confidence interval: 33.9-81.3; TA 35.5% [5.2-65.9]; TS 90.9% [73.9-100]). Survival up to four years according to vascular access showed a clear benefit in patients treated transseptally (p=0.045).
Conclusions: Regardless of the access route, TMVIV/TMVIR was associated with high technical success yet impaired device success. In the long term, TA access had a significant adverse impact on survival.
Introduction
Mitral regurgitation (MR) is the most prevalent valvular heart disease in adults1. The predominant surgical treatment options are mitral valve repair with annuloplasty rings in addition to various resection techniques or the implantation of neochordae and mitral valve replacement with bioprosthetic tissue valves. Recurrence of moderate or severe MR within two years of surgical repair has been reported in 59% of cases2. Surgically implanted bioprostheses have limited durability and may degenerate such that stenosis, regurgitation or both recur3. Repeat surgery in patients originally treated with mitral valve repair or replacement is often associated with a high risk, particularly in elderly patients with comorbidities4.
Currently, transcatheter aortic valve replacement (TAVR) is also a therapeutic option for patients with degenerated aortic valve bioprostheses (“valve-in-valve” procedure)5,6. The valve-in-valve concept has been extended to the mitral valve, with transcatheter aortic valve prostheses implanted in degenerated mitral valve bioprostheses or failed mitral valve annuloplasty rings7,8.
The present study focused on outcomes of transcatheter mitral valve-in-valve implantation (TMVIV) for degenerated mitral valve bioprostheses, and transcatheter mitral valve-in-ring implantation (TMVIR) for failed annuloplasty rings, according to the recently published Mitral Valve Academic Research Consortium (MVARC) criteria9, stratified by the access route.
Methods
PATIENTS
Between November 2010 and September 2015, 24 consecutive patients (72±13 years; eight men [33%]) underwent TMVIV (n=14) or TMVIR (n=10) procedures. Prior to the intervention, the therapeutic approach had been discussed within a dedicated Heart Team. The indication for the procedure was the presence of MR (n=17) or mitral stenosis (n=7) (Figure 1).
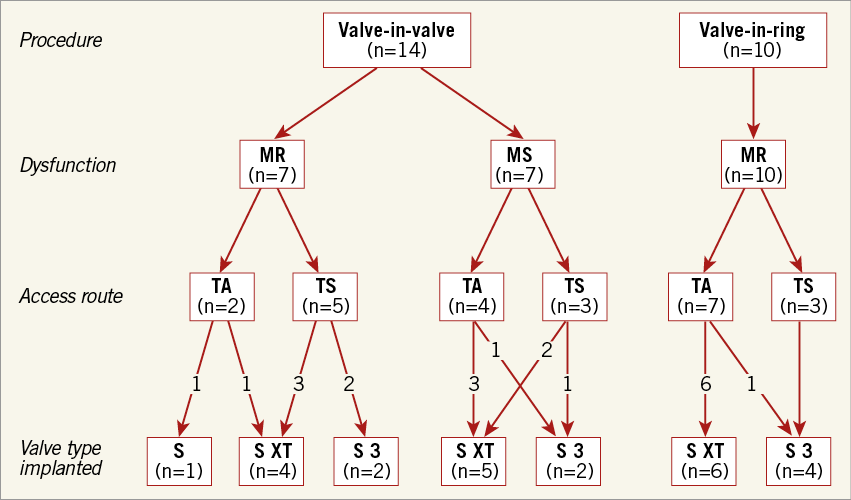
Figure 1. Flow chart of patients according to type of procedure, type of mitral valve/ring dysfunction, access route, and type of aortic valve prosthesis implanted. MR: mitral regurgitation; MS: mitral stenosis; S: SAPIEN; TA: transapical; TS: transseptal
TMVIV/TMVIR PROCEDURE
All procedures were performed in a hybrid operating room. Patients referred by their general practitioner to our hospital’s Department of Cardiac Surgery underwent TMVIV/TMVIR by way of transapical (TA) access, whereas patients referred to the Department of Cardiology underwent transseptal (TS) TMVIV/TMVIR. Details of the different vascular access techniques have previously been described10,11.
For sizing of the TAVR prosthesis, the internal diameter of the degenerated bioprosthesis or failed annuloplasty ring was checked against the reported diameter of the manufacturer and by using the mitral valve-in-valve app12. In addition, all diameters were measured by transoesophageal echocardiography (TEE). Outcome parameters were classified according to the MVARC criteria9.
INVASIVE HAEMODYNAMICS
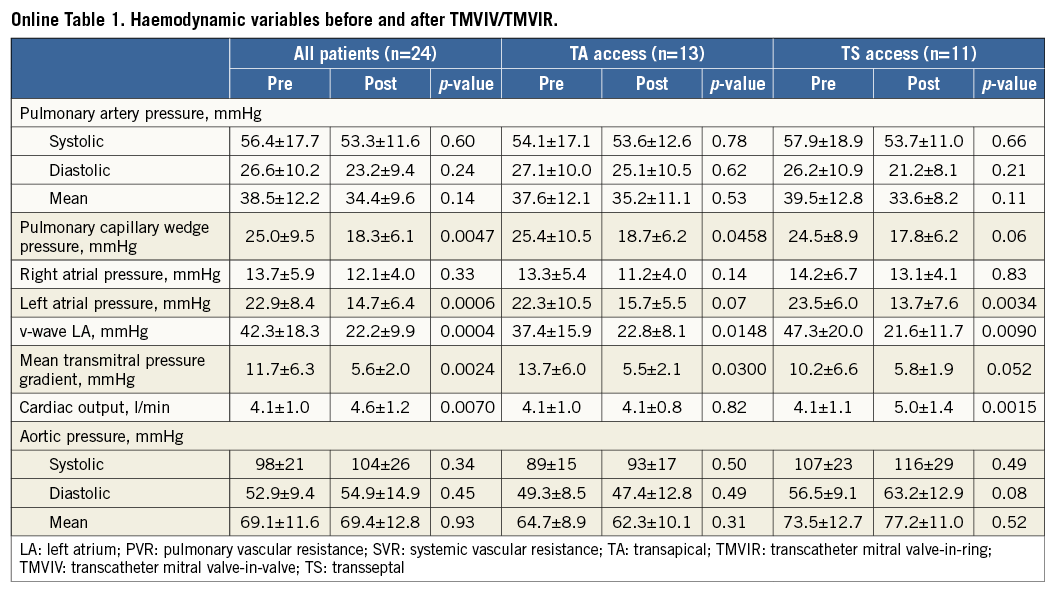
Right heart catheterisation was performed in all patients with a 7 Fr Swan-Ganz™ catheter (Edwards Lifesciences, Irvine, CA, USA) before and after TMVIV/TMVIR. Right atrial pressure, systolic, diastolic and mean pulmonary artery pressures, as well as pulmonary capillary wedge and left atrial pressures, were recorded. Cardiac output was determined using the thermodilution method.
FOLLOW-UP
After hospital discharge, follow-up was scheduled at 30 days, six and 12 months, and annually thereafter.
ETHICS
Written informed consent was obtained from all patients or their representatives.
STATISTICAL ANALYSIS
Continuous variables are described as means and standard deviations if normally distributed, or as medians plus interquartile range if not. Differences between continuous variables were analysed with t-tests or the Mann-Whitney U test where appropriate. Categorical variables are described with absolute and relative frequencies. Differences between categorical variables were evaluated with the chi-square test or Fisher’s exact test. Survival curves were estimated by the Kaplan-Meier method. A two-tailed p-value <0.05 was considered statistically significant.
Results
PATIENTS
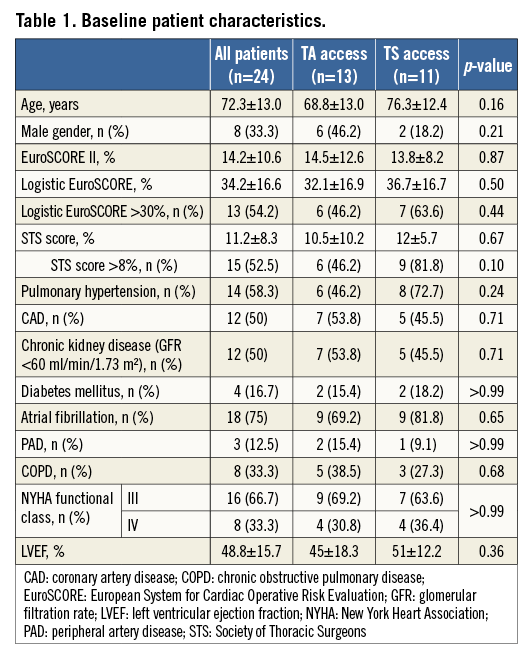
All 24 patients were severely symptomatic, with dyspnoea of New York Heart Association (NYHA) functional Class III (n=16) or IV (n=8), and at high surgical risk; mean logistic EuroSCORE was 34%, mean EuroSCORE II 14%, and mean Society of Thoracic Surgeons (STS) score 11%. The patients’ baseline characteristics are given in Table 1.
PROCEDURES
Transapical (TA) access was chosen in 13, and transseptal (TS) access in 11 patients (Figure 1). The latter approach was performed either via a transfemoral (n=10) or transjugular (n=1) venous route. There was no difference in the choice of access between the first 12 patients, who were treated between 2010 and 2012 (TA n=7, TS n=5), and the last 12 patients who were treated between 2013 and 2015 (TA n=6, TS n=6).
Aortic valve prostheses used in this study were the balloon-expandable SAPIEN (n=1), SAPIEN XT (n=15) and SAPIEN 3 (n=8) bioprostheses (Edwards Lifesciences, Irvine, CA, USA). General anaesthesia was used in 21 patients (87.5%); three TS patients were treated under a mild analgosedation despite using TEE during the intervention due to the anaesthesiologist’s wish. Pre-balloon mitral valvuloplasty was performed in three patients (12.5%) (TA n=1, TS n=2) with severely stenotic bioprostheses. In two of these three patients, a cerebral protection device (Claret Medical, Santa Rosa, CA, USA) was used; in the other patient, the cerebral protection device could not be inserted due to adverse anatomy of the truncus brachiocephalicus.
TMVIV/TMVIR was performed at 8.8±4.4 (range 1-16) years after the index surgery. The interventions lasted for a median of 180 (IQR 120-205) minutes, with no statistically significant difference between TA access (median 150 [IQR 120-180] minutes) and TS access (median 180 [IQR 128-248] minutes, p=0.16). Most time-consuming in TA procedures were access problems due to adhesions; crossing the septum and positioning the delivery catheter within the mitral valve were the most time-consuming steps during TS procedures.
In addition to TMVIV, two patients (#7, #12) underwent regular TAVR for concomitant severe aortic stenosis. Two other patients (#8, #14) who underwent TMVIR had, in addition, an Amplatzer™ Vascular Plug III (St. Jude Medical, St. Paul, MN, USA) implanted to close a para-annular leak of the mitral annuloplasty ring due to partial detachment of the ring (Moving image 1-Moving image 5). Both patients were initially treated for severe functional MR with annular dilatation by downsizing of the annulus with a surgical annuloplasty ring. One of these patients also underwent neochordae implantation using a GORE-TEX® suture (W.L. Gore, Flagstaff, AZ, USA).
ACUTE OUTCOMES
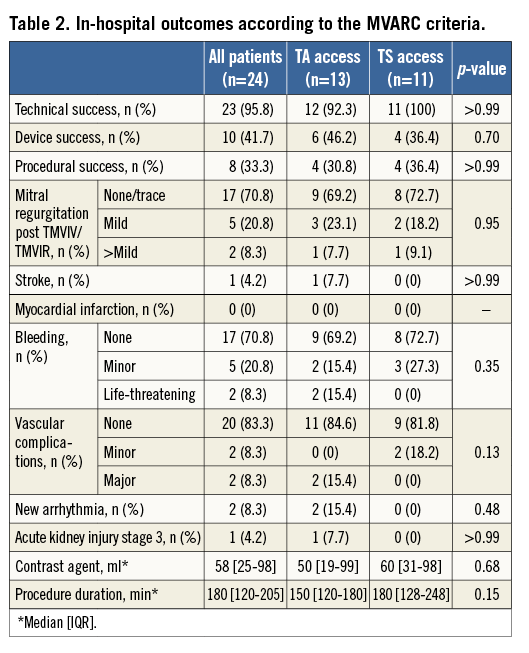
MVARC technical success (assessed at discharge from the hybrid operating theatre) was 95.8%. There was one technical success failure in the TA group due to the need for a second bioprosthesis. The reason for that was persistent MR due to the first transcatheter prosthesis being implanted “too deep” towards the left ventricle, missing the covered part of the transcatheter prosthesis. The second transcatheter prosthesis was implanted within the first prosthesis “higher” towards the left atrium.
Post-dilatation was required in 11 patients (45.8%) (TMVIV n=5, TMVIR n=6) to reduce persistent paravalvular regurgitation. Post-dilatation was successful in all cases except for one TMVIR patient in whom mild-to-moderate paravalvular MR persisted. After TMVIV/TMVIR implantation, no obstruction of the left ventricular outflow tract was seen by echocardiography in any patient. No specific screening was carried out before the procedure to estimate the risk of outflow tract obstruction. MVARC device success (assessed at 30 days) was achieved in 10 patients (41.7%; TA 46.2% vs. TS 36.4%, p=0.70). Reasons for device failure were a mean post-interventional transmitral pressure gradient ≥5 mmHg (n=13 [54%]) and greater than mild MR (n=1) at discharge.
There were 11 patients (45.8%) with a transmitral pressure gradient ≤5 mmHg, 13 (54.2%) with a gradient between 6 and 10 mmHg, and no patient with a gradient >10 mmHg. The patients with a post-interventional transprosthetic gradient between 6 and 10 mmHg had pre-existing mitral bioprostheses and rings in seven and six cases, respectively. The type of degeneration was regurgitation and stenosis in nine and four patients, respectively. Pre- and post-procedural transprosthetic gradients available in 12 of the 13 patients revealed an intervention-related decrease by -7.2±4.8 mmHg in eight patients and an increase by 3.3±1.5 mmHg in four.
MVARC procedural success (assessed at 30 days) was 33.3% (n=8). Reasons for procedural failure were the lack of device success (n=14), the occurrence of a major vascular complication, life-threatening bleeding, acute kidney injury, and stroke in one patient, as well as a non-cardiac death in another patient.
TA patients had a longer median [IQR] in-hospital stay than TS patients (11 [9-17] days vs. 7 [4.5-8] days, p=0.0099).
INVASIVE HAEMODYNAMICS
Right heart catheterisation revealed significant decreases after TMVIV/TMVIR in left atrial and pulmonary capillary wedge pressures, as well as in the left atrial v-wave and the mean transmitral pressure gradient; cardiac output increased significantly (Online Table 1). These changes were also present, or reflected (i.e., just missing statistical significance), in the patient subgroups, except for cardiac output. This variable increased significantly (by 1.1±0.8 l/min) only in patients treated via the TS approach; in contrast, there was no change in cardiac output in patients undergoing TA treatment (0.0±0.5 l/min; p=0.0051 vs. TS).
PERIPROCEDURAL COMPLICATIONS
One patient (#12) sustained a stroke immediately after the procedure. The patient had a severely stenotic mitral valve bioprosthesis and was treated by TA TMVIV without predilatation, followed in the same session by transfemoral TAVR (23 mm CoreValve®; Medtronic, Minneapolis, MN, USA) for a 21 mm Epic™ valve (St. Jude Medical).
Of the 11 patients who underwent TS TMVIV/TMVIR, one had a drop in oxygenation after removal of the transcatheter system from the septum due to a significant residual atrial septal defect that was treated by an Amplatzer™ Septal Occluder device (St. Jude Medical). Crossing the septum in this patient with the delivery catheter took some time and manipulation. Furthermore, the patient had pulmonary hypertension, reflected by a systolic pulmonary artery pressure of 65 mmHg. Two of the 13 TA procedures had a major vascular complication due to problems with the apical access and need for blood transfusion. Detailed outcome parameters are shown in Table 2.
FOLLOW-UP
Patients were followed for a median of 19 (IQR 4-39) months (TA: 20 [IQR 3-29] months vs. TS: 12 [IQR 6-49] months, p=0.43). Overall 30-day mortality was 12.5% (TA 15.4% and TS 9.1%), since three patients died five, 11 and 23 days after the procedure. The early death after five days was due to a cardiovascular cause. This 72-year-old female patient had been treated as an emergency case due to cardiogenic shock with multiple organ dysfunction; she had required extracorporeal membrane oxygenation after successful TMVIV. The other two patients died of pneumonia. A further five patients died more than 30 days after the intervention; the cause of death was cardiovascular in two of these patients. Overall three-year survival was estimated at 57.6% (95% confidence interval, 33.9-81.3%; TA 35.5% [5.2-65.9%]; TS 90.9% [73.9-100%]).
Survival up to four years according to vascular access showed a benefit in patients treated transseptally (Figure 2). The longest follow-up was 57.5 months in a 67-year-old female patient treated via TS access who presented in NYHA functional Class II at the time of her follow-up visit.
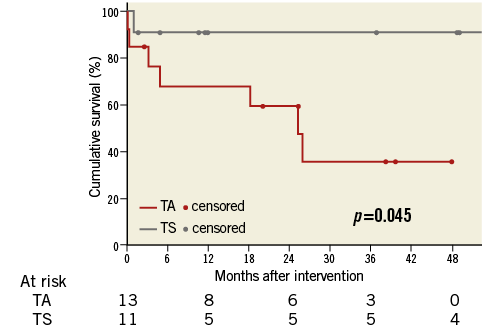
Figure 2. Cumulative survival according to vascular access route.
Discussion
In 2015, a consensus paper by the MVARC was published to ensure universal use of endpoint definitions regarding safety and efficacy of MR therapies9. By then, several case series with different endpoint definitions had been published for TMVIV/TMVIR procedures7,13. Our data on 24 high surgical risk patients treated with TMVIV/TMVIR implantation are the first to report outcome variables according to access route and MVARC definitions.
MAIN FINDINGS
The main findings of this study are:
TMVIV/TMVIR using three different balloon-expandable bioprostheses and either TA or TS access is feasible;
with just one periprocedural stroke occurring in a patient undergoing TA TMVIV, the transcatheter technique appeared to be reasonably safe;
TMVIV/TMVIR resulted in significant decreases in left atrial and pulmonary capillary wedge pressures, in the left atrial v-wave and the mean transmitral pressure gradient, indicative of a successful reduction of left atrial volume overload;
in patients treated via the TS approach – but not in patients treated via TA access – TMVIV/TMVIR caused a significant increase in cardiac output;
TS as opposed to the TA approach was associated with improved survival.
TA VS. TS ACCESS
Data from the Valve-in-Valve International Data (VIVID) registry including 437 patients showed that TA access is the preferred route (78.9% of patients). In contrast, in our study, only 54% of patients were treated via TA access. The TA approach with its direct, shortest, and co-axial access to the mitral valve has advantages with respect to positioning and implantation of the new bioprosthesis. However, patients treated with TA access in our series showed no acute improvement in cardiac output. Moreover, survival in these patients was worse than in patients treated via TS access.
The higher mortality for TA patients in our series advocates a primary treatment strategy via TS access, particularly in patients with an excessively high surgical risk, as reflected in a logistic EuroSCORE >30%. Le Ven et al showed a direct correlation of cardiac output and outcome of patients after TAVR14. Patients with a stroke volume index ≥35 ml/m² measured within five days of TAVR had a better outcome than patients in whom that index was <30 ml/m²; moreover, the TA approach for TAVR was associated with a lower “early” post-procedural stroke volume index (p<0.001)14. This finding is consistent with our results for TA patients.
To date, no study has compared outcomes of the TA vs. the TS access route. TA access is still a surgical approach which requires a left mini-thoracotomy. The less invasive TS approach is technically more challenging in order to achieve coaxial alignment of the new prosthesis with the degenerated surgical bioprosthesis or ring. However, no difference in procedure times between patients treated by either approach was seen in our series.
OUTCOME
TMVIV/TMVIR results for a time period exceeding three months are limited to seven reports with a total of 93 patients15. The mortality rate after a mean follow-up of 14 months was 20.5%16. Ye et al showed data with a median follow-up of 2.5 years and a maximum of eight years16. Patients in our study were followed for a median of 19 months, with a one-year estimated mortality rate of 22%. Most of the previously published results for TMVIV/TMVIR procedures had a procedural success rate of 100%, despite a residual transmitral pressure gradient ≥5 mmHg15. This is in contrast to our overall procedural success of 33%; the striking difference is most likely due to the fact that our procedural success rate was calculated on the basis of the MVARC criteria. To facilitate comparisons, future endpoint definitions should use those criteria.
PROCEDURAL ASPECTS
The predominant reason for device failure was the persistence of a mean post-interventional transmitral pressure gradient ≥5 mmHg in 13 patients. A recent meta-analysis of 113 patients treated by TMVIV/TMVIR procedures reported a mean gradient of 6.3 mmHg after implantation15, which appears to be concordant with our study (5.6 mmHg). Data from the VIVID registry also showed an elevated mean gradient of 5.9 mmHg after TMVIV/TMVIR procedures. Even surgically implanted bioprostheses have a mean transmitral gradient of up to 6.3 mmHg immediately after the operation17.
The mean transmitral pressure gradient cut-off of ≥5 mmHg indicating significant mitral stenosis is generally used for transcatheter mitral valve repair procedures such as MitraClip® (Abbott Vascular, Santa Clara, CA, USA) implantation. Patients enrolled in the 2011-2012 Pilot European Sentinel Registry had a mean transmitral pressure gradient of 3.4 mmHg after the MitraClip procedure18. On the other hand, in the past, procedural success for MitraClip procedures was defined as a reduction in MR to grade ≤2+18,19. MR severity ≤2+ post TMVIV/TMVIR was achieved in all our study patients. However, we had one patient with device failure due to persistence of mild-to-moderate MR after the procedure. It is important to have consensus endpoint definitions for all valve procedures because otherwise comparisons of results from different studies will not be possible. The MVARC counted the 5 mmHg transmitral pressure gradient cut-off among those endpoint variables for which “further studies are warranted to determine the prognostic effect of these measures”9.
In all cases TEE was used for guidance. Three TS patients were treated without general anaesthesia. Due to the duration of the procedure with a TEE probe in the oesophagus, most patients were intubated. This is in accordance with MitraClip procedures19. However, in selected patients the procedure can be done safely without general anaesthesia20. With growing experience in TS mitral valve procedures one might forego general anaesthesia. In contrast to previous reports, embolisation of the new bioprosthesis was not observed in our study21,22.
Pizzarello et al observed an association of the height of the left atrial v-wave with the presence of acute and chronic MR23. In line with these results, our haemodynamic measurements also showed significant decreases in left atrial pressure and the left atrial v-wave as signs of a successful treatment of the degenerated bioprosthesis or failed ring.
Balloon valvuloplasty pre-implantation was only carried out in three cases (17%) because of the risk of debris embolisation and stroke. As in other reports, implantation without pre-ballooning was safe and feasible24.
Limitations
This is an observational study with a limited number of patients from a single centre. Patients were not assigned to TA or TS access on the grounds of predefined clinical, haemodynamic, or echocardiographic criteria; rather, access choice was determined by the hospital department to which they were referred. In addition, the data represent a retrospective analysis without randomisation. Patient success at one year according to MVARC could not be assessed because of missing rehospitalisation rates.
Conclusions
This study presents a retrospective single-centre comparison of the two predominant access routes used for TMVIV/TMVIR procedures. Mortality was lower for patients treated by TS rather than TA access. This finding might change the current access strategy for TMVIV/TMVIR procedures. A procedure-related increase in cardiac output was only seen in TS patients. Our findings need to be verified in larger patient cohorts.
According to the MVARC criteria, TMVIV/TMVIR procedures were associated with a high technical success rate. However, due to a mean transmitral pressure gradient ≥5 mmHg persisting after the intervention in 54% of our patients, the device success rate was low. The 5 mmHg cut-off should be discussed for a potential update of the MVARC criteria in the future.
| Impact on daily practice TMVIV/TMVIR procedures are feasible via the TS as well as the TA approach. However, the current access strategy may change in favour of the TS approach because of the lower mortality observed in patients treated by TS access and an increase in cardiac output observed only in TS, not in TA patients. |
Conflict of interest statement
C. Frerker has received grants from Edwards Lifesciences. K-H. Kuck has received grants and consultation fees from St. Jude Medical, Biosense Webster, and Medtronic, outside of the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Transoesophageal echocardiography at baseline with valvular as well as para-ring mitral regurgitation.
Moving image 2. 3D transoesophageal echocardiography at baseline with valvular as well as para-ring (5-6 o’clock) mitral regurgitation.
Moving image 3. Transoesophageal echocardiography after implantation of a plug with still severe valvular mitral regurgitation.
Moving image 4. 3D transoesophageal echocardiography after implantation of a plug with still severe valvular mitral regurgitation.
Moving image 5. Final transoesophageal echocardiography after TMVIR and plug implantation.
Supplementary data
To read the full content of this article, please download the PDF.
Transoesophageal echocardiography at baseline with valvular as well as para-ring mitral regurgitation.
3D transoesophageal echocardiography at baseline with valvular as well as para-ring (5-6 o’clock) mitral regurgitation.
Transoesophageal echocardiography after im plantation of a plug with still severe valvular mitral regurgitation.
3D transoesophageal echocardiography after implantation of a plug with still severe valvular mitral regurgitation.
Final transoesophageal echocardiography after TMVIR and plug implantation.

