Abstract
Background: Transcatheter mitral valve replacement (TMVR) using dedicated devices is an alternative therapy for high-risk patients with symptomatic mitral regurgitation (MR).
Aims: This study aimed to assess the 2-year outcomes and predictors of mortality in patients undergoing TMVR from the multicentre CHOICE-MI Registry.
Methods: The CHOICE-MI Registry included consecutive patients with symptomatic MR treated with 11 different dedicated TMVR devices at 31 international centres. The investigated endpoints included mortality and heart failure hospitalisation rates, procedural complications, residual MR, and functional status. Multivariable Cox regression analysis was applied to identify independent predictors of 2-year mortality.
Results: A total of 400 patients, median age 76 years (interquartile range [IQR] 71, 81), 59.5% male, EuroSCORE II 6.2% (IQR 3.8, 12.0), underwent TMVR. Technical success was achieved in 95.2% of patients. MR reduction to ≤1+ was observed in 95.2% at discharge with durable results at 1 and 2 years. New York Heart Association Functional Class had improved significantly at 1 and 2 years. All-cause mortality was 9.2% at 30 days, 27.9% at 1 year and 38.1% at 2 years after TMVR. Chronic obstructive pulmonary disease, reduced glomerular filtration rate, and low serum albumin were independent predictors of 2-year mortality. Among the 30-day complications, left ventricular outflow tract obstruction, access site and bleeding complications showed the strongest impact on 2-year mortality.
Conclusions: In this real-world registry of patients with symptomatic MR undergoing TMVR, treatment with TMVR was associated with a durable resolution of MR and significant functional improvement at 2 years. Two-year mortality was 38.1%. Optimised patient selection and improved access site management are mandatory to improve outcomes.
Introduction
Mitral regurgitation (MR) is the most common valvular disease in industrialised countries, with increasing prevalence in elderly patients12. Traditionally, the treatment of patients with severe MR has relied on surgical repair or replacement, especially in patients with primary MR34. However, a substantial portion of patients with MR are not referred to surgery due to high or prohibitive risk5. For these patients, transcatheter edge-to-edge repair (TEER) is a feasible and effective alternative6. Nonetheless, eligibility for TEER may be limited because of unfavourable mitral valve anatomy or the potential risk of postprocedural mitral stenosis7. Furthermore, residual or recurrent MR can occur in a considerable portion of patients undergoing TEER, with increased rates of mortality and heart failure (HF) hospitalisations8910.
Transcatheter mitral valve replacement (TMVR) using dedicated mitral valve prostheses has evolved into a complementary treatment option for patients ineligible for surgery or TEER11. Unlike other transcatheter strategies, the main advantage of TMVR is complete resolution of MR in the majority of patients. Recent early feasibility studies with different TMVR devices reported encouraging outcomes with high technical success and low procedural mortality121314. However, published outcomes after TMVR are currently limited to experience with single devices and small sample sizes. Data on large-scale outcomes and predictors of outcome are scarce.
Based on data from the multicentre CHoice of OptImal transCatheter trEatment for Mitral Insufficiency Registry (CHOICE-MI; ClinicalTrials.gov: NCT04688190), we sought to investigate characteristics, outcomes and predictors of mortality in patients undergoing TMVR with different transapical (TA) and transfemoral (TF) devices.
Methods
Study design and study population
The study design of the CHOICE-MI Registry has been described in detail previously11. In brief, this retrospective, multicentre registry included all consecutive patients with symptomatic MR who underwent screening for TMVR eligibility at 33 participating international centres from May 2014 to July 2022. For the present study, patients with TMVR screening failure were excluded and only patients undergoing TMVR were included (Figure 1). A detailed list of participating centres is given in Supplementary Table 1. All procedures were planned and performed by the local interdisciplinary Heart Teams. Baseline and follow-up data obtained by the individual centres were centrally stored and analysed. There was no event adjudication committee. The study was performed in accordance with the Declaration of Helsinki and with the approval of the local institutional review boards.
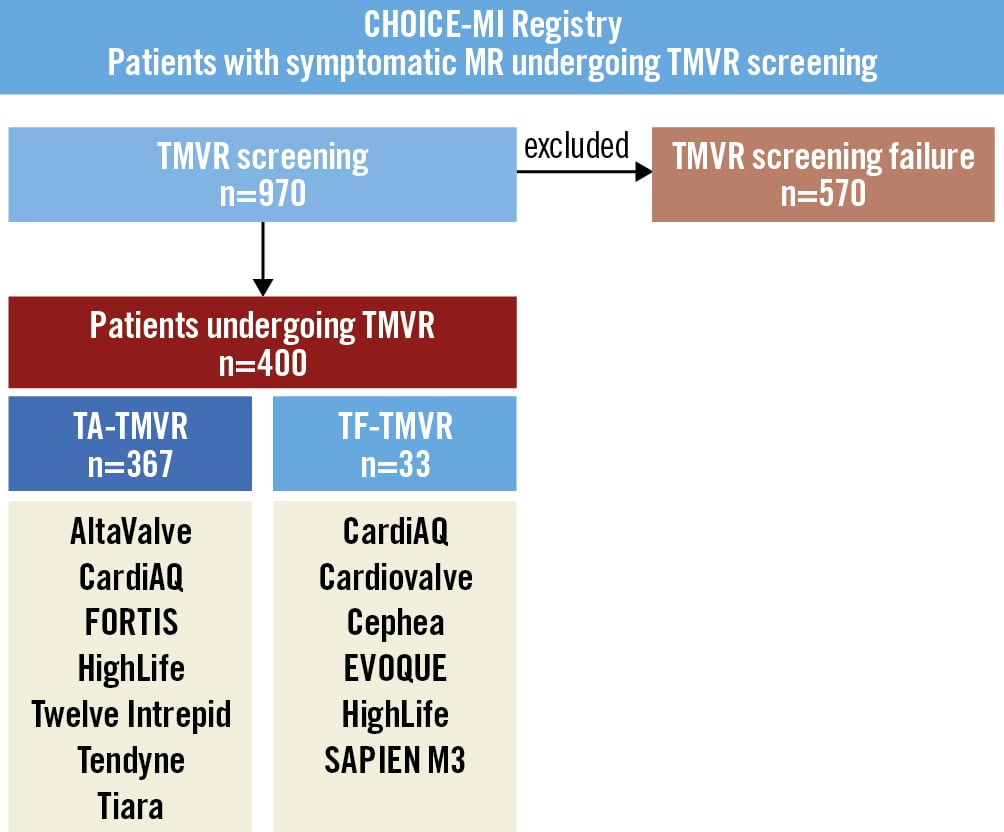
Figure 1. Study flowchart. The CHOICE-MI Registry included all consecutive patients undergoing TMVR screening (N=970). This study included only patients undergoing TMVR with different transapical or transfemoral devices (N=400). Patients with TMVR screening failure (N=570) were excluded for this analysis. MR: mitral regurgitation; TA: transapical; TF: transfemoral; TMVR: transcatheter mitral valve replacement
Transcatheter mitral valve replacement devices
Patients in this study were treated with 11 different TMVR devices with either a TA or TF approach: AltaValve (4C Medical Technologies), CardiAQ (Edwards Lifesciences), Cardiovalve (Venus Medtech), Cephea (Abbott), EVOQUE (Edwards Lifesciences), FORTIS (Edwards Lifesciences), HighLife (HighLife SAS), Twelve Intrepid (Medtronic), SAPIEN M3 (Edwards Lifesciences), Tendyne (Abbott), and Tiara (Neovasc Inc.). The procedural steps and implantation techniques for each device have been described previously. Clinical and anatomical eligibility for TMVR was evaluated according to device-specific protocols.
Echocardiographic assessment
The aetiology and severity of MR were assessed at each participating site in accordance with current guidelines. MR aetiology was defined as either primary, secondary, or mixed primary and secondary MR. MR severity at baseline, discharge, and follow-up was graded as none or trivial, mild (1+), moderate (2+), moderate-to-severe (3+), or severe (4+). The assessment of MR included the effective regurgitant orifice area (EROA), regurgitant volume, and mean mitral transvalvular pressure gradient (MPG). Further echocardiographic measurements included the left atrial volume (LAV), stroke volume index (SVI), left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD) and volume (LVEDV), and left ventricular end-systolic diameter (LVESD) and volume (LVESV). Tricuspid annular plane systolic excursion (TAPSE), pulmonary artery systolic pressure (PASP), and tricuspid regurgitation severity (TR) were measured to evaluate right ventricular (RV) function.
Computed tomography assessment
All patients underwent multislice computed tomography (CT) as part of the screening process. The CT variables included measuring the mitral annular dimensions (intercommissural [CC] diameter, anteroposterior [AP] diameter, annulus perimeter and area), aortomitral angulation and severity of mitral annulus calcification (MAC). MAC severity was graded as mild, moderate, or severe, according to Eleid and colleagues1516. In this study, MAC severity was dichotomised (
Study endpoints
The primary study endpoint was defined as all-cause mortality at 2 years. Secondary study endpoints included cardiovascular (CV) mortality, the incidence of HF hospitalisation and the combined endpoint of all-cause mortality or HF hospitalisation at 2 years. Residual MR was assessed at discharge, and at 1 and 2 years. Left ventricular (LV) and RV parameters were measured at 1 and 2 years. Functional status, determined by New York Heart Association (NYHA) Functional Class, was assessed at 1- and 2-year follow-ups. Technical and device success and in-hospital and 30-day complication rates were reported according to the Mitral Valve Academic Research Consortium (MVARC) definitions17. Procedural mortality was defined as patient death in the operating room.
Statistical analysis
Continuous variables are shown as medians with interquartile range (IQR). Binary variables are shown as counts (frequencies). Differences between timepoints were tested by the paired Mann-Whitney U-test for continuous variables and by the McNemar test for categorical variables. The median follow-up time was calculated using the reverse Kaplan-Meier estimator. The survival probabilities of patients were estimated using the Kaplan-Meier method. Univariable Cox regression was conducted to identify baseline characteristics associated with 2-year all-cause mortality. All variables with a p-value <0.25 in univariable Cox regression were chosen for multivariable selection methods. A backward subset selection was performed using a method that selected the model based on Akaike information criterion (AIC)18. To prepare the subset selection, variables with a high rate of missing values (>20%) or high correlation (>0.8) were excluded to ensure a valid selection for the multivariable Cox regression. Hazard ratios (HR) and 95% confidence intervals (CI) are presented for all parameters. The association between the procedural and 30-day MVARC complications and the primary endpoint were calculated by Cox regression and adjusted for parameters in the multivariable Cox regression model (i.e., diabetes, coronary artery disease [CAD], chronic obstructive pulmonary disease [COPD], estimated glomerular filtration rate [eGFR] and serum albumin <3.3 g/dl). The results are displayed in a forest plot. A p-value <0.05 was considered statistically significant. All analyses were performed with R statistical software version 4.0.3 (R Foundation for Statistical Computing).
Results
Baseline characteristics
Out of a total of 970 patients undergoing TMVR screening, 400 patients underwent TMVR with 11 different dedicated devices at 31 centres in Europe (n=26), North America (n=4), and Australia (n=1). Most patients underwent TA-TMVR (n=367), while 33 patients underwent TF-TMVR. The detailed numbers of patients treated per each device are summarised in Supplementary Table 2. Supplementary Table 3 gives an overview of ongoing trials for each device.
Baseline clinical characteristics of all TMVR patients are given in Table 1. The TMVR study population (median age 76.0 years [IQR 71.0, 81.0], 59.5% [n=238] male, European System for Cardiac Operative Risk Evaluation [EuroSCORE] II 6.2% [IQR 3.8, 12.0]) is characterised by a high prevalence of cardiac (e.g., atrial fibrillation and CAD) and non-cardiac comorbidities (e.g., renal dysfunction). Most patients were severely symptomatic, being in NYHA Functional Class III or IV (83.2%, n=333) with at least one HF hospitalisation in the previous 12 months prior to TMVR treatment in 70.2% (n=275) of all patients.
Table 2 shows echocardiographic and CT measurements at baseline. The aetiology of MR was secondary in 195 patients (50.0%), primary in 127 (32.6%), and mixed in 68 patients (17.4%). The median LVEF was 45% (IQR 35, 55), and 31 patients (8.1%) had an LVEF <30%. RV dysfunction (TAPSE <17 mm) and pulmonary hypertension (PASP >50 mmHg) were prevalent in 47.9% and 38.7%, respectively, of patients undergoing TMVR. Moderate or severe MAC was found in 20.4% (n=57/280) of patients with available CT data.
Table 1. Baseline clinical characteristics.
| Parameter | All TMVR (n=400) |
|---|---|
| Baseline clinical parameters | |
| Age, years | 76.0 (71.0-81.0) |
| Male sex | 238 (59.5) |
| BMI, kg/m2 | 25.6 (22.5-28.7) |
| EuroSCORE II, % | 6.2 (3.8-12.0) |
| STS-PROM (MV replacement), % | 5.9 (4.1-8.7) |
| STS-PROM (MV repair), % | 3.7 (2.3-5.8) |
| Atrial fibrillation | 233 (66.2) |
| Hypertension | 293 (74.9) |
| Diabetes | 114 (28.5) |
| Extracardiac arteriopathy | 84 (21.5) |
| Coronary artery disease | 220 (62.5) |
| Prior myocardial infarction | 138 (35.2) |
| Prior CABG | 119 (29.8) |
| Prior PCI | 161 (40.2) |
| COPD | 73 (18.2) |
| Prior stroke | 49 (12.2) |
| eGFR <60 ml/min/1.73 m2 | 286 (73.7) |
| Prior dialysis | 15 (3.9) |
| Serum albumin <3.3 g/dl | 45 (14.0) |
| HF hospitalisation (prior 12 months) | 275 (70.2) |
| NYHA Functional Class III/IV | 333 (83.2) |
| NT-proBNP, pg/ml | 2,623.0 (1,184.7-5,673.0) |
| Heart failure medication | |
| Beta blocker | 296 (85.3) |
| ACE-inhibitor/ARB | 241 (69.1) |
| ARNI | 46 (13.9) |
| MRA | 143 (44.8) |
| SGLT-2 inhibitor | 25 (8.9) |
| Data are n (%) or median (IQR). ACE: angiotensin converting enzyme; ARB: angiotensin II receptor blocker; ARNI: angiotensin receptor-neprilysin inhibitor; BMI: body mass index; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; EuroSCORE: European System for Cardiac Operative Risk Evaluation; HF: heart failure; IQR: interquartile range; MRA: mineralocorticoid receptor antagonists; MV: mitral valve; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SGLT-2: sodium-glucose co-transporter 2; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TMVR: transcatheter mitral valve replacement | |
Table 2. Echocardiographic and CT parameters at baseline.
| Parameter | All TMVR (n=400) |
|---|---|
| Echocardiography | |
| Primary MR | 127 (32.6) |
| Secondary MR | 195 (50.0) |
| Mixed primary/secondary MR | 68 (17.4) |
| Mitral stenosis (severe) | 14 (4.3) |
| MPG, mmHg | 3.0 (2.0-4.3) |
| EROA, mm² | 0.40 (0.27-0.49) |
| RVol, ml | 54.0 (40.0-70.0) |
| LAV, ml | 102.9 (77.0-135.9) |
| LVEF, % | 45.0 (35.0-55.0) |
| LVEF <30% | 31 (8.1) |
| LVEDD, mm | 58.0 (52.0-63.0) |
| LVEDV, ml | 150.0 (116.1-197.8) |
| LVESD, mm | 44.0 (37.1-51.0) |
| LVESV, ml | 84.0 (56.0-122.0) |
| SVI, ml/m2 | 32.2 (25.6-39.9) |
| PASP, mmHg | 48.0 (39.0-60.0) |
| PASP >50 mmHg | 139 (38.7) |
| TAPSE, mm | 17.0 (13.0-20.0) |
| TAPSE <17 mm | 156 (47.9) |
| TR ≥3+ | 64 (18.8) |
| Computed tomography | |
| CC diameter, mm | 39.8 (36.6-42.5) |
| AP diameter, mm | 32.4 (29.1-36.3) |
| Annulus perimeter, mm | 121.9 (111.0-130.0) |
| Annulus area, cm2 | 11.1 (9.4-12.7) |
| Aortomitral angulation, ° | 130.5 (124.9-136.2) |
| MAC (≥moderate) | 57 (20.4) |
| Predicted neo-LVOT area, mm2 | 409.0 (310.0-497.0) |
| Data are n (%) or median (IQR). AP: anteroposterior; CC: intercommissural; CT: computed tomography; EROA: effective regurgitant orifice area; IQR: interquartile range; LAV: left atrial volume; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume; LVOT: left ventricular outflow tract; MAC: mitral annulus calcification; MPG: mean transvalvular pressure gradient; MR: mitral regurgitation; PASP: pulmonary artery systolic pressure; RVol: regurgitant volume; SVI: stroke volume index; TAPSE: tricuspid annular plane systolic excursion; TMVR: transcatheter mitral valve replacement; TR: tricuspid regurgitation | |
Procedural and MVARC 30-day outcomes
Procedural complications and MVARC 30-day outcomes are shown in Figure 2. Among all patients undergoing TMVR, MVARC-defined technical success was achieved in 381 patients (95.2%). Four patients died in the operating room (1.0%), and conversion to open heart surgery was required in 9 patients (2.4%). Acute left ventricular outflow tract (LVOT) obstruction occurred in 15 patients (3.8%) (Figure 2A). To evaluate the learning curve of centres, we compared procedural outcomes between the early experience group (i.e., the first device implantations at each center, N=54) and the late experience group (i.e., subsequent implantations, N=346). There was a trend towards lower procedural mortality in the late experience group (3.9% vs 0.6%, p=0.087), whereas no differences were found regarding technical success (98.2% vs 94.8%; p=0.49) or conversion to open heart surgery (2.1% vs 2.4%; p=1.00) (Supplementary Table 4).
Among MVARC-defined 30-day endpoints, the highest event rate (14.8%) was observed for acute kidney injury (Acute Kidney Injury Network [AKIN] stage 2 or 3). Major access site-related complications and major or worse (i.e., extensive, life-threatening or fatal) bleeding occurred in 5.5% and 11.2% of patients, respectively. Low rates were found for myocardial infarction (0.6%) and disabling stroke (3.1%). A total of 36 patients died within the first 30 days after TMVR (Kaplan-Meier estimated event rate 9.2%) − the majority from cardiovascular causes (n=30/36, 83.3%) (Figure 2B).
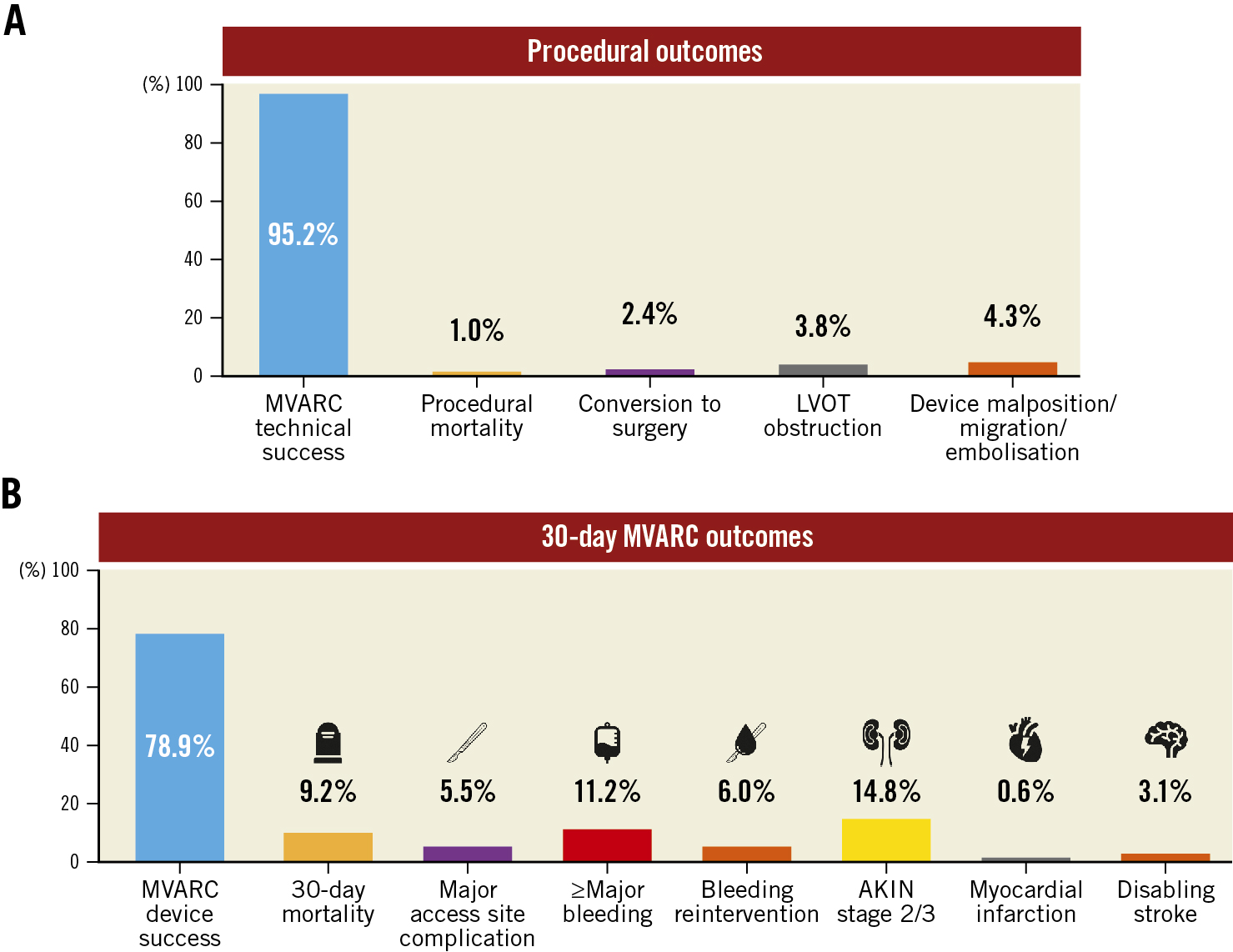
Figure 2. Procedural and 30-day MVARC outcomes. Procedural outcomes after TMVR (A) and 30-day MVARC outcomes (B) are summarised in the barplots. Each bar depicts the respective event rate (%). Overall complication rates were low, with low procedural mortality and a high technical success rate. AKIN: Acute Kidney Injury Network; LVOT: left ventricular outflow tract; MVARC: Mitral Valve Academic Research Consortium; TMVR: transcatheter mitral valve replacement
Echocardiographic outcomes
MR was eliminated (i.e., none or trace residual MR) following TMVR in 85.9% of patients at discharge and reduced to MR ≤1+ in 95.2%. The rates of MR elimination and reduction to MR ≤1+ were stable at follow-up (73.7% and 94.2%, respectively, at 1 year, and 75.0% and 94.6%, respectively, at 2 years; all p<0.001 compared to baseline) (Figure 3A). A bar plot showing residual MR, including the rates of death and missing data (where MR was not assessed, or the follow-up time has not yet been reached), is given in Supplementary Figure 1A.
Paired echocardiographic parameters for patients with available 1- and 2-year follow-up after TMVR are presented in Table 3. A durable reduction of MR after 1 and 2 years was observed in more than 90% of patients with successful MR reduction at discharge. There was a significant reduction in LVEF (−2.5% [IQR −10.0, 2.0]; p<0.001) and a statistical trend towards lower LVEDD (−1.2 mm [IQR −5.0, 3.0]; p=0.054) after 1 year, but not after 2 years. A reduction in PASP was observed after 1 year (−10 mmHg [IQR −22.0, 2.8]; p<0.001) and after 2 years (−13.0 mmHg [IQR −24.8, −3.0]; p<0.001), while a reduction in the rate of patients with TR ≥3+ was observed only after 1 year (−21 patients; p<0.001), with a statistical trend after 2 years (−6 patients; p=0.077). No significant changes were found for MPG, LVESD, LVEDV, LVESV or TAPSE at 1 or 2 years.
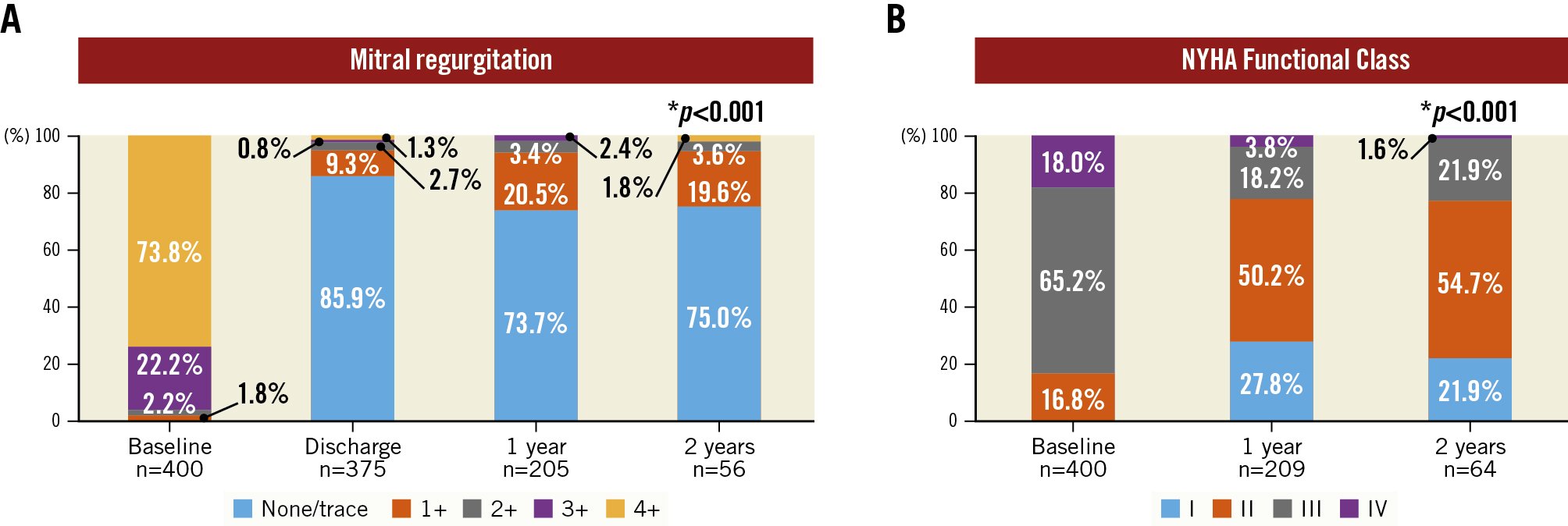
Figure 3. Mitral regurgitation and functional outcomes. Mitral regurgitation (MR) (A) and New York Heart Association (NYHA) Functional Class (B) at baseline and follow-up are presented in barplots. Compared to baseline, both MR (discharge, 1- and 2-year follow-up) and NYHA Functional Class (1- and 2-year follow-up) improved significantly. *p<0.001 for the comparison between baseline versus discharge (only for MR), baseline versus 1-year follow-up (MR and NYHA), and baseline versus 2-year follow-up (MR and NYHA)
Table 3. Paired echocardiographic outcomes at 1- and 2-year follow-up after TMVR.
| Baseline vs 1-year follow-up | |||||
|---|---|---|---|---|---|
| Echocardiography endpoints | Paired samples (n) | Baseline | 1-year follow-up | Change from baseline | p-value |
| MR 2+ or lower, (%) | 205 | 3 (1.5) | 200 (97.6) | 197 (96.1) | <0.001 |
| MR 1+ or lower, (%) | 205 | 1 (0.5) | 193 (94.1) | 192 (93.7) | <0.001 |
| MPG, mmHg | 133 | 3.0 (2.0, 4.0) | 3.0 (2.2, 4.4) | 0 (‒1.0, 2.0) | 0.080 |
| LVEF, % | 190 | 42.0 (35.0, 54.0) | 40.0 (30.0, 50.0) | ‒2.5 (‒10.0, 2.0) | <0.001 |
| LVEDD, mm | 114 | 59.0 (52.0, 64.0) | 58.0 (50.9, 63.1) | ‒1.2 (‒5.0, 3.0) | 0.054 |
| LVEDV, ml | 62 | 144.0 (119.6, 187.6) | 146.4 (98.4, 200.7) | ‒2.5 (‒40.0, 24.7) | 0.65 |
| LVESD, mm | 97 | 45.0 (39.5, 52.0) | 46.0 (37.0, 56.4) | 0.9 (‒6.3, 7.2) | 0.61 |
| LVESV, ml | 56 | 76.6 (51.2, 120.0) | 79.9 (54.4, 140.0) | 4.5 (‒15.8, 28.0) | 0.23 |
| TAPSE, mm | 118 | 16.0 (12.0, 19.1) | 15.0 (12.0, 19.0) | ‒1.0 (‒4.0, 3.0) | 0.20 |
| PASP, mmHg | 127 | 48.0 (40.2, 61.7) | 40.0 (33.0, 47.0) | ‒10.0 (‒22.0, 2.8) | <0.001 |
| TR ≥3+, (%) | 153 | 35 (8.79) | 14 (3.52) | 21 (13.7) | <0.001 |
| Baseline vs 2-year follow-up | |||||
| Echocardiography endpoints | Paired samples (n) | Baseline | 2-year follow-up | Change from baseline | p-value |
| MR 2+ or lower, (%) | 56 | 0 (0) | 55 (98.2) | 55 (98.2) | n/a |
| MR 1+ or lower, (%) | 56 | 0 (0) | 53 (94.6) | 53 (94.6) | n/a |
| MPG, mmHg | 38 | 2.2 (2.0, 4.0) | 3.1 (2.4, 4.0) | 1.0 (‒1.0, 2.0) | 0.14 |
| LVEF, % | 53 | 39.0 (32.7, 54.3) | 35.0 (30.0, 53.0) | 0.0 (‒7.3, 4.3) | 0.18 |
| LVEDD, mm | 41 | 61.0 (53.0, 65.3) | 62.0 (55.7, 66.0) | 1.0 (‒4.0, 4.3) | 0.55 |
| LVEDV, ml | 27 | 167.0 (123.1, 198.5) | 165.0 (98.2, 235.9) | ‒8.0 (‒31.8, 31.0) | 0.91 |
| LVESD, mm | 29 | 47.0 (40.0, 54.3) | 52.0 (43.3, 55.3) | 2.0 (‒3.3, 10.3) | 0.21 |
| LVESV, ml | 24 | 98.6 (59.3, 125.8) | 112.5 (57.7, 164.9) | 1.0 (‒14.5, 39.7) | 0.20 |
| TAPSE, mm | 35 | 17.0 (12.0, 19.8) | 15.0 (11.2, 19.0) | ‒2.0 (‒3.8, 1.8) | 0.11 |
| PASP, mmHg | 43 | 50.0 (42.3, 60.0) | 36.0 (29.0, 44.8) | ‒13.0 (‒24.8, -3.0) | <0.001 |
| TR ≥3+, (%) | 44 | 9 (2.26) | 3 (0.75) | 6 (13.6) | 0.077 |
| Data are n (%) or median (IQR). LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume; MPG: mean transvalvular pressure gradient; MR: mitral regurgitation; n/a: not available; PASP: pulmonary artery systolic pressure; TAPSE: tricuspid annular plane systolic excursion; TMVR: transcatheter mitral valve replacement; TR: tricuspid regurgitation | |||||
Functional outcomes
Functional outcome according to NYHA Functional Class is demonstrated in Figure 3B. At baseline, 333 patients (83.2%) were in NYHA Functional Class III or IV; at 1-year follow-up, 78.0% of patients (n=163/209 patients) were in NYHA Functional Class I or II. This rate remained stable (76.6%, n=49/64) in patients with available 2-year follow-up. A bar plot showing NYHA Functional Class, including the rates of death and missing data, is presented in Supplementary Figure 1B.
Mortality and heart failure hospitalisation
A total of 137 deaths occurred over a median follow-up of 1.34 years (95% CI: 1.13-1.65) and a maximum follow-up of 7.29 years. Figure 4 displays 2-year Kaplan-Meier survival curves for the primary endpoint of all-cause mortality and the secondary endpoints of cardiovascular mortality and the composite of all-cause mortality or incident HF hospitalisation. The rates of all-cause mortality after TMVR were 27.9% and 38.1% at 1 and 2 years, respectively (Figure 4A). There were no differences in 2-year all-cause mortality according to MR aetiology (primary MR 38.8%, secondary MR 38.2%, mixed 38.7%; p=0.47) or TMVR access (TA 38.4%, TF 35.1%; p=0.93). In addition, 2-year mortality did not differ between patients undergoing TMVR with the most frequently implanted device (37.9%) versus all other TMVR devices (39.2%; p=0.68) (Supplementary Figure 2). Inspection of the survival curve suggested an elevated event rate until approximately 30 days after TMVR. In a landmark analysis excluding early events up to 30 days after the procedure, mortality rates were 20.6% at 1 year and 31.8% at 2 years (Figure 4B); cardiovascular mortality accounted for 19.2% and 22.0% at 1 and 2 years, respectively (Figure 4C).
The reported rates of the combined endpoint of all-cause mortality or incident HF hospitalisation were 38.5% at 1 year and 49.0% at 2 years. Flattening of the Kaplan-Meier curve for the combined endpoint was observed at approximately 4 months after the procedure.
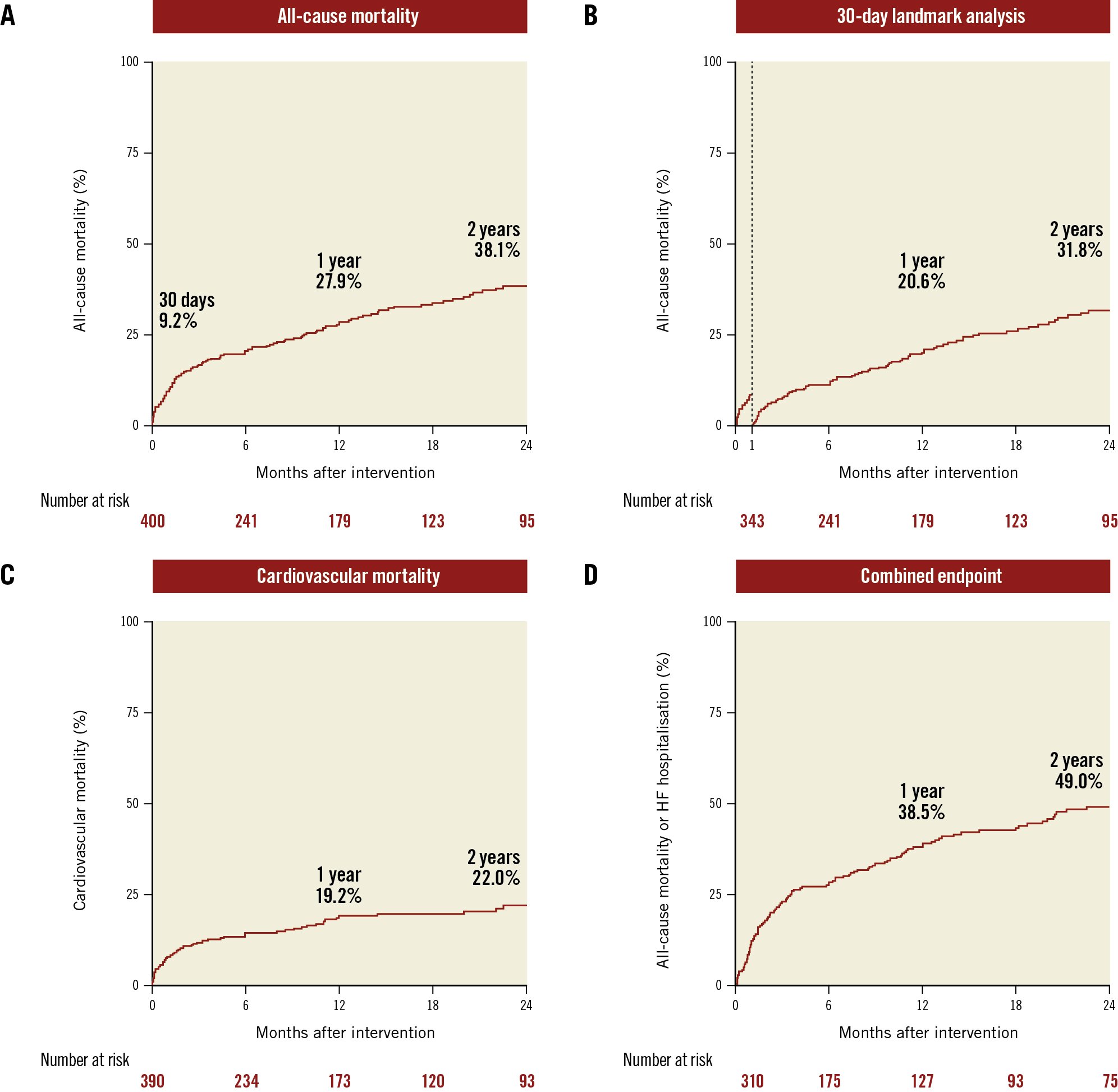
Figure 4. Primary and secondary study endpoints. Kaplan-Meier curves are shown for all-cause mortality after 2 years (A), a 30-day landmark analysis for all-cause mortality after 2 years (B), cardiovascular mortality after 2 years (C) and the combined endpoint of all-cause mortality or incident heart failure hospitalisation (D).
Device-related events
During follow-up, TMVR device thrombosis was reported in 17 patients (5.7%) and infective endocarditis was observed in 10 patients (3.4%). A total of 20 patients (6.5%) underwent mitral valve reintervention, including both transcatheter and surgical mitral valve reinterventions.
Predictors of mortality after TMVR
Univariable and multivariable Cox regression analysis for 2-year all-cause mortality using backward subset selection by AIC are given in Table 4. The final multivariable model included CAD, COPD, diabetes, eGFR, and serum albumin <3.3 g/dl. Among these variables, serum albumin <3.3 g/dl (HR 2.27, 95% CI: 1.42-3.62; p<0.001), COPD (HR 1.75, 95% CI: 1.08-2.82; p=0.022), and eGFR (HR 0.99, 95% CI: 0.98-1.00; p=0.008) were independent predictors of 2-year all-cause mortality after TMVR. In an additional Cox regression analysis focusing on predictors of long-term survival by excluding events within 30 days after TMVR, only COPD (HR 1.73, 95% CI: 1.02-2.95; p=0.042) and eGFR (HR 0.98, 95% CI: 0.97-1.00; p=0.001) remained independently associated with 2-year all-cause mortality (Supplementary Table 5).
Table 4. Univariable and multivariable Cox regression analysis for all-cause mortality at 2 years.
| Univariable (n=400) | Multivariable (n=283) | |||
|---|---|---|---|---|
| Parameters | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value |
| Age | 1.01 (0.99-1.04) | 0.29 | ||
| Female sex | 1.06 (0.73-1.53) | 0.76 | ||
| Body mass index, kg/m2 | 1.01 (0.97-1.05) | 0.74 | ||
| Arterial hypertension | 1.29 (0.83-1.99) | 0.26 | ||
| Atrial fibrillation | 1.00 (0.67-1.51) | 0.99 | ||
| Coronary artery disease | 0.77 (0.52-1.14) | 0.19 | 0.73 (0.47-1.12) | 0.15 |
| Extracardiac arteriopathy | 1.23 (0.80-1.90) | 0.35 | ||
| COPD | 1.61 (1.06-2.45) | 0.026 | 1.75 (1.08-2.82) | 0.022 |
| Diabetes | 1.51 (1.03-2.22) | 0.036 | 1.51 (0.96-2.37) | 0.072 |
| Prior CABG | 0.74 (0.49-1.11) | 0.14 | ||
| Prior PCI | 0.98 (0.68-1.43) | 0.93 | ||
| Prior myocardial infarction | 0.82 (0.56-1.20) | 0.30 | ||
| Prior stroke | 1.18 (0.69-2.03) | 0.55 | ||
| Prior dialysis | 1.86 (0.87-4.00) | 0.11 | ||
| eGFR, ml/min/1.73 m² | 0.98 (0.97-0.99) | <0.001 | 0.99 (0.98-1.00) | 0.008 |
| Serum albumin <3.3 g/dl | 2.33 (1.50-3.62) | <0.001 | 2.27 (1.42-3.62) | <0.001 |
| NYHA Functional Class III/IV | 1.06 (0.64-1.75) | 0.83 | ||
| MR aetiology (secondary MR) | 0.84 (0.58-1.22) | 0.36 | ||
| EROA, cm2 | 1.29 (0.65-2.57) | 0.46 | ||
| Regurgitant volume, ml | 1.00 (0.99-1.01) | 0.95 | ||
| MPG, mmHg | 1.03 (0.95-1.13) | 0.43 | ||
| LVEF, % | 1.00 (0.98-1.01) | 0.80 | ||
| SVI, ml/m2 | 1.01 (0.99-1.03) | 0.28 | ||
| LVEDD, mm | 0.99 (0.96-1.01) | 0.20 | ||
| LVEDV, ml | 1.00 (1.00-1.00) | 0.73 | ||
| LVESD, mm | 1.00 (0.98-1.03) | 0.69 | ||
| LVESV, ml | 1.00 (1.00-1.01) | 0.73 | ||
| LAV, ml | 1.00 (1.00-1.01) | 0.22 | ||
| TAPSE, mm | 1.00 (0.96-1.04) | 0.92 | ||
| PASP, mmHg | 1.00 (0.99-1.01) | 0.99 | ||
| TR ≥2+ | 0.91 (0.61-1.35) | 0.63 | ||
| ≥moderate MAC | 1.09 (0.63-1.89) | 0.76 | ||
| CABG: coronary artery bypass graft; CI: confidence interval; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; EROA: effective regurgitant orifice area; LAV: left atrial volume; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume; MAC: mitral annulus calcification; MPG: mean transvalvular pressure gradient; MR: mitral regurgitation; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; PCI: percutaneous coronary intervention; SVI: stroke volume index; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation | ||||
Prognostic association of 30-day complications with mortality
The adjusted association of procedural and 30-day MVARC complications with 2-year all-cause mortality is presented in a forest plot (Figure 5). Regarding periprocedural complications, the strongest association with 2-year mortality was found for LVOT obstruction (HR 4.03, 95% CI: 1.46-9.10; p=0.01). Major access site complications (HR 3.38, 95% CI: 1.58-6.50; p=0.003), major or worse bleeding (HR 2.85, 95% CI: 1.56-4.92; p=0.001), and acute renal failure AKIN stage 2 or 3 (HR 2.44, 95% CI: 1.44-3.96; p=0.0012) were identified as MVARC 30-day complications that were strongly associated with 2-year all-cause mortality.
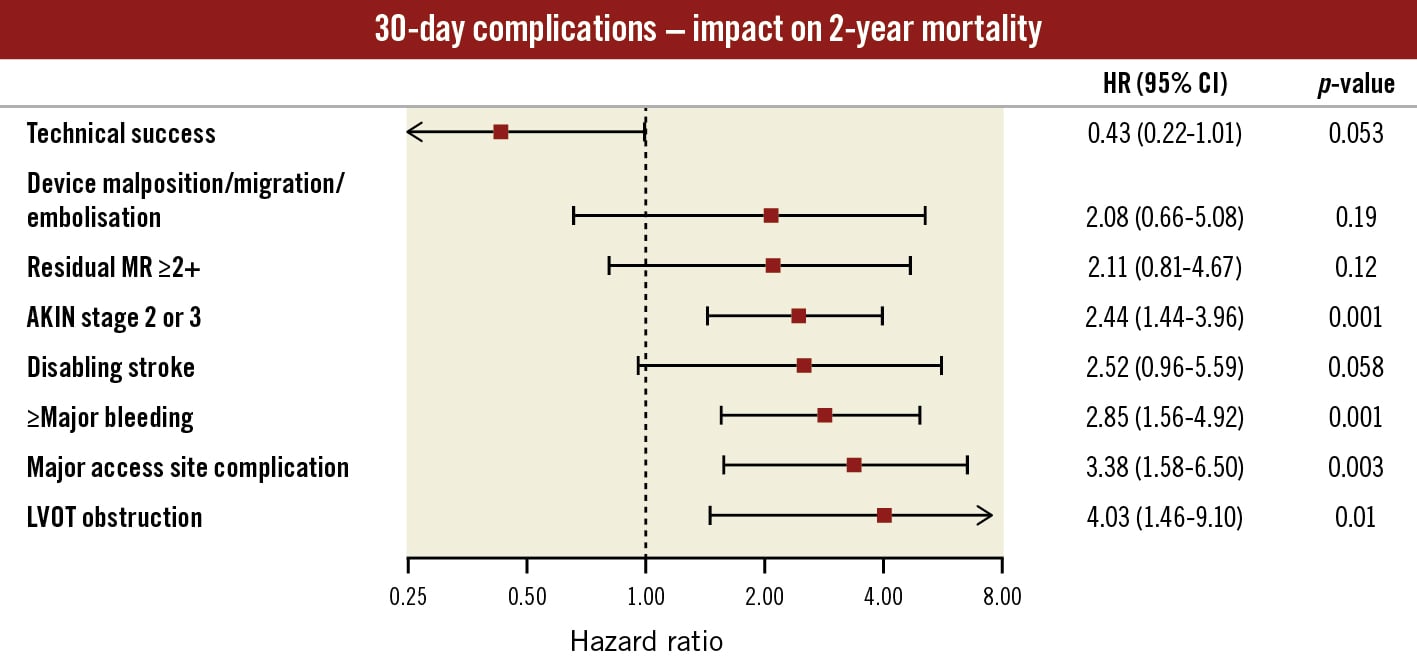
Figure 5. Adjusted impact of 30-day complications on 2-year all-cause mortality. Cox regression for 2-year all-cause mortality was performed for procedural complications and 30-day MVARC-defined outcomes adjusted for baseline predictors from the multivariable model (CAD, COPD, diabetes, eGFR, serum albumin <3.3 g/dl; Table 4). Adjusted hazard ratios are displayed in a forest plot. A clipped CI is indicated by arrows. The strongest association with 2-year all-cause mortality was found for LVOT obstruction, access site and bleeding complications. AKIN: Acute Kidney Injury Network; CAD: coronary artery disease; CI: confidence interval; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; HR: hazard ratio; LVOT: left ventricular outflow obstruction; MR: mitral regurgitation; MVARC: Mitral Valve Academic Research Consortium
Discussion
This study reports the outcomes of 400 consecutive patients undergoing TMVR enrolled in the international, multicentre CHOICE-MI Registry with follow-up for 2 years. This is by far the largest data collection on this topic reported to date. Given the limited data available on outcomes after TMVR, this study provides important insights into the potential advantages, expected outcomes and future directions of this therapy (Central illustration):
1) TMVR procedures were technically successful in the vast majority of patients, and procedural complication rates, including procedural mortality, were low.
2) Treatment with TMVR resulted in predictable resolution of MR with durable results at 2 years. Paired echocardiographic outcomes demonstrated significant reductions in PASP, TR severity, and LVEF at follow-up.
3) Rates of mortality and HF hospitalisation at 2 years appeared to be largely attributable to elevated early event rates followed by a flattened survival curve. This timepoint was approximately 1 month after TMVR for all-cause mortality and approximately 4 months for HF hospitalisation.
4) Low serum albumin, COPD and reduced renal function were found to be independent predictors of 2-year all-cause mortality, while only COPD and reduced renal function remained predictive when excluding events occurring within 30 days after TMVR. This highlights the need for careful patient selection regarding TMVR candidacy. Among MVARC 30-day complications, access site-related and bleeding complications were strongly associated with 2-year mortality.
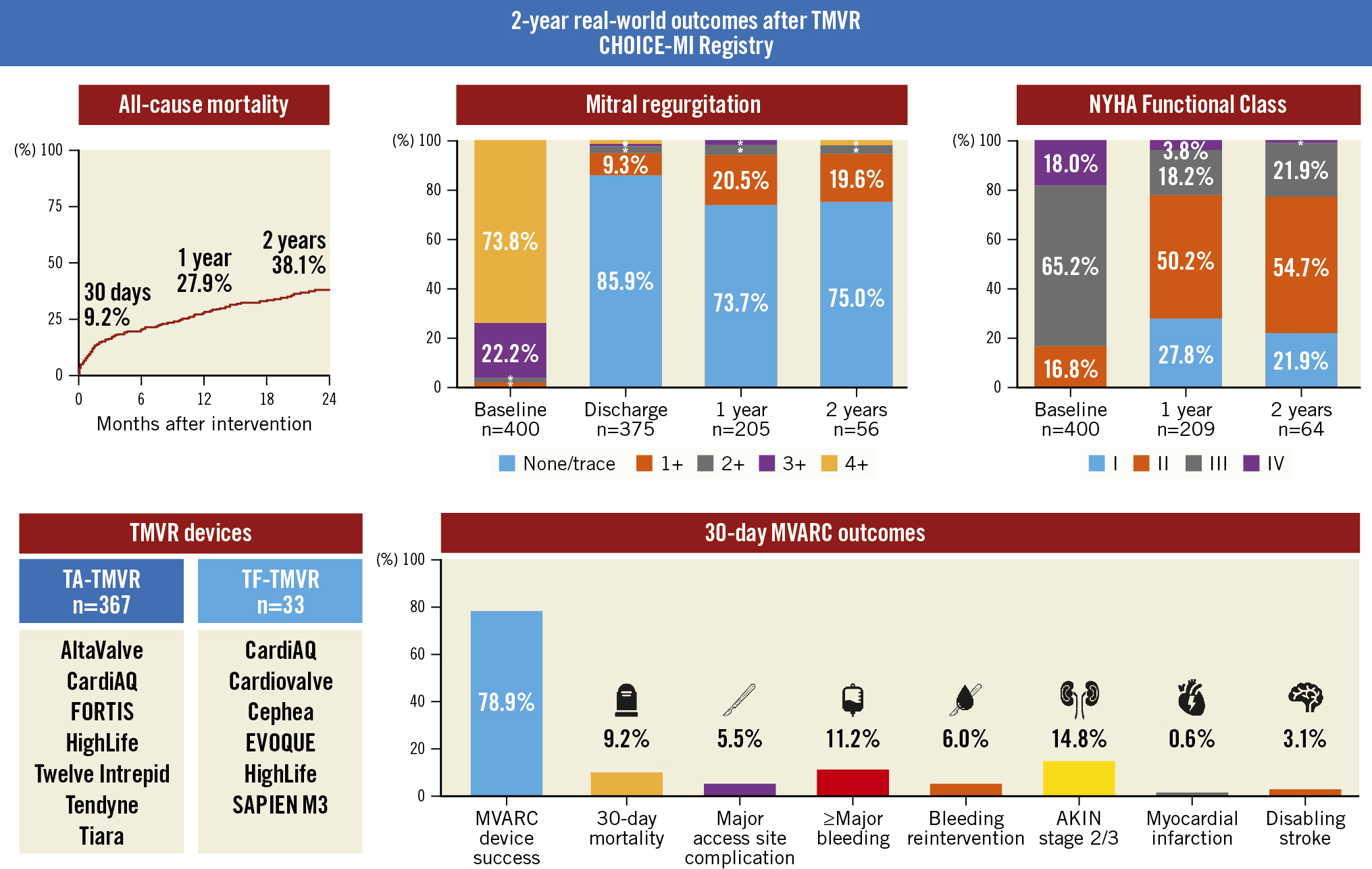
Central illustration. Real-world outcomes after TMVR − results from the CHOICE-MI Registry. Clinical and echocardiographic outcomes of 400 consecutive patients undergoing TMVR with 11 different devices were investigated. Treatment with TMVR was associated with a predictable and durable resolution of MR and functional improvement in the majority of patients. AKIN: Acute Kidney Injury Network; CHOICE-MI: CHoice of OptImal transCatheter trEatment for Mitral Insufficiency; MVARC: Mitral Valve Academic Research Consortium; NYHA: New York Heart Association; TA: transapical; TF: transfemoral; TMVR: transcatheter mitral valve replacement
TMVR candidacy and future directions
Patients with symptomatic MR considered for TMVR in real-world practice represent a challenging subset of patients given their high prevalence of comorbidities, elevated surgical risk, and complex anatomical features that preclude treatment with more established therapies1119. Transcatheter implantation of bioprosthetic valves holds the promise of being a reproducible, safe and effective solution for these patients. This study presents encouraging data, showing high rates of technical success, low rates of procedural complications, and predictable MR elimination after TMVR for this high-risk group of patients. Yet, the initial experience with TMVR was associated with high rates of screening failure, allowing few patients access to TMVR treatment and leaving the majority to bailout strategies or medical therapy alone2021. Aside from prohibitive annular dimensions − outside the available treatment ranges − the risk of periprocedural LVOT obstruction was a major reason for the high screening failure rate, and indeed, the present study reinforces the significance of LVOT obstruction as a rare, but potentially fatal, complication with limited treatment options after final device deployment. The identification of patients at risk for LVOT obstruction is somewhat complex, but the actual number of patients at risk might be lower than anticipated. For example, one study showed that measuring the neo-LVOT in early systole may better discriminate the risk of LVOT obstruction compared with end-systolic estimates, thereby increasing the number of potential candidates22. While there is agreement that patients with secondary MR and elevated LV volumes generally have a lower risk of LVOT obstruction, our study suggests increasing real-world rates for primary and mixed MR aetiology, even in just the last year11. Future generations of TMVR devices are expected to focus on minimising the risk of LVOT obstruction by lowering ventricular device profiles and optimising the interaction with the anterior mitral valve leaflet. Reducing the risk of LVOT obstruction, in addition to emerging access routes, anchoring approaches, and broader availability of device sizes, is expected to increase the number of patients amenable to TMVR over the coming years.
Rationale and impact of MR elimination
While the most established mitral valve therapies (TEER and surgical mitral valve repair) offer effective and reliable treatment for most patients with significant MR, residual and recurrent MR remain unsolved issues, especially in patients with secondary MR. Several studies have shown that both residual and recurrent MR occur in a considerable portion of these patients and are associated with increased rates of mortality9102324. Elimination of MR represents the central rationale behind the concept of TMVR. In line with previous studies, the present study confirms predictable complete resolution of MR in the vast majority of patients, with durable results and functional improvement at follow-up12132526. Furthermore, TMVR was associated with significant reductions in pulmonary artery pressure and TR severity. While reductions in LVEF have already been shown after both TEER and TMVR1227, which can be explained by the common overestimation of LVEF in the presence of MR and the increased afterload following MR treatment, the reduction in LVEDD at follow-up and the potential impact of MR elimination on LV remodelling after TMVR warrant further investigation.
Determinants of TMVR outcomes
With a mortality rate of 38% at 2 years following TMVR, determining the factors associated with impaired survival in these patients is important. The only available data come from a multivariate analysis in a small subcohort (n=51) of the Tendyne Global Feasibility Study. Badhwar et al identified baseline MR severity, prior percutaneous coronary intervention, renal function and arterial hypertension as independent predictors of 1-year outcome28. Based on a large real-world TMVR cohort, the present study identified low serum albumin, COPD, and renal function as independent predictors of 2-year all-cause mortality, while echocardiographic parameters had no impact on outcomes after TMVR, suggesting that long-term survival in patients undergoing TMVR is mainly determined by non-cardiac comorbidities. These results highlight a potential to improve outcomes by optimising not just the anatomical but also the clinical patient selection for TMVR. This becomes even more important concerning the choice of access. While the Tendyne TMVR system (Abbott) is the only European conformity (CE)-marked device to date, most TMVR procedures are performed via TA access. Similar to the history of transcatheter aortic valve replacement, frail patients and those with a high burden of comorbidities appear to tolerate TA access less well in terms of mortality and hospital readmissions, compared to the less traumatic TF approach2930. Indeed, this study identified access site and bleeding complications after TMVR as those postprocedural complications showing the strongest association with 2-year mortality. The expected transition to transfemoral devices might provide a solution for some of these issues31. Recently, Zahr et al published the first outcome data from the Intrepid Transfemoral TMVR Early Feasibility Study. Despite promising results regarding 30-day survival, stroke and reintervention rates, major vascular complications and major or worse bleeding events occurred in 47% and 40% of treated patients, respectively14. The authors concluded that a large-bore delivery sheath, comorbidities and the need for anticoagulation might explain these findings. To improve the long-term survival of patients undergoing TMVR, optimising access management is crucial, regardless of the delivery route. In this study, we did not find a significant difference in 2-year mortality with regard to TF- or TA-TMVR. However, the small number of patients undergoing TF-TMVR that were included in our study only allows for a limited interpretation of this finding. Results from ongoing studies with multiple TF-TMVR devices will be important to evaluate the potential advantages of TF over TA access in TMVR.
Study limitations
This study is mainly limited by its study design as a retrospective registry. All results can, therefore, only help generate hypotheses. Although a detailed comparison between TA- and TF-TMVR would have been desirable, this analysis did not seem feasible at the time, given the small number of patients treated with TF-TMVR devices. Moreover, conclusions regarding functional outcome without a medical control group available should be drawn cautiously. Finally, clinical and echocardiographic follow-up was incomplete, and there was no echocardiographic core laboratory analysis, which especially imposes limits on the interpretation of paired echocardiographic outcomes. However, the CHOICE-MI Registry is the largest collection of patients undergoing TMVR with different devices to date and should assist understanding of both current and future roles of TMVR for the treatment of patients with MR. The results of ongoing randomised controlled trials comparing TMVR to TEER (e.g., SUMMIT, ClinicalTrials.gov: NCT03433274) will help define its role in comparison to established MR therapies.
Conclusions
Based on data from the international CHOICE-MI Registry, this study investigated 2-year outcomes of 400 patients undergoing TMVR with different TA- and TF-TMVR devices. Treatment with TMVR was associated with a durable resolution of MR and functional improvement at follow-up. Baseline non-cardiac comorbidities were identified as independent predictors of 2-year mortality, while access site and bleeding complications showed the strongest association with mortality among the postprocedural complications. Optimised patient selection and access site management will be important factors to improve outcomes after TMVR.
Impact on daily practice
Transcatheter mitral valve replacement (TMVR) represents an alternative therapy for selected high-risk surgical patients with mitral regurgitation (MR). Based on a large collection of patients undergoing TMVR with dedicated devices, our study suggests effectiveness and functional improvement up to 2-year follow-up after TMVR, while patient selection and access site management were the key determinants of mortality. Randomised controlled trials, long-term follow-up and data on outcomes with transfemoral TMVR devices are warranted to define the future role of TMVR among established MR therapies.
Appendix. Study collaborators
Stefan Blankenberg, MD; Department of Cardiology, University Heart & Vascular Center Hamburg, Hamburg, Germany and German Center for Cardiovascular Research (DZHK), Partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Benedikt Koell, MD; Department of Cardiology, University Heart & Vascular Center Hamburg, Hamburg, Germany and German Center for Cardiovascular Research (DZHK), Partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; Niklas Schofer, MD; Department of Cardiology, University Heart & Vascular Center Hamburg, Hamburg, Germany and German Center for Cardiovascular Research (DZHK), Partner site Hamburg/Kiel/Lübeck, Hamburg, Germany; André Vincentelli, MD, PhD; CHU Lille, Institut Pasteur de Lille, Lille, France; Arnaud Sudre, MD; CHU Lille, Institut Pasteur de Lille, Lille, France; John G. Webb, MD; St. Paul’s Hospital, Vancouver, BC, Canada; Philipp Blanke, MD; St. Paul’s Hospital, Vancouver, BC, Canada; Marcel Weber, MD; Heart Center Bonn, Bonn, Germany; Tetsu Tanaka, MD; Heart Center Bonn, Bonn, Germany; Johanna Vogelhuber, MD; Heart Center Bonn, Bonn, Germany; Mirjam Wild, MD; Medizinische Klinik und Poliklinik I, Klinikum der Universität München, Munich, Germany; Rüdiger Lange, MD; Department of Cardiovascular Surgery, German Heart Center Munich, Munich, Germany and INSURE – Institute for Translational Cardiac Surgery, Department of Cardiovascular Surgery, German Heart Centre Munich, Germany; Laurin Ochs, MD; Department of Cardiology, Heart Center, University Hospital Cologne, Cologne, Germany; Elmar Kuhn, MD; Department of Cardiology, Heart Center, University Hospital Cologne, Cologne, Germany; Cristina Giannini, MD, PhD; Cardiac Catheterization Laboratory, Cardiothoracic and Vascular Department, University of Pisa, Pisa, Italy; Marco De Carlo, MD, PhD; Cardiac Catheterization Laboratory, Cardiothoracic and Vascular Department, University of Pisa, Pisa, Italy; Didier Tchétché, MD; Groupe CardioVasculaire Interventionnel, Clinique Pasteur, Toulouse, France; Marco Metra, MD; Cardiac Catheterization Laboratory and Cardiology, ASST Spedali Civili di Brescia, Brescia, Italy and Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Brescia, Italy; Francesco Bedogni, MD; IRCCS Policlinico San Donato, Milan, Italy; Christian Frerker, MD; Medical Clinic II, University Heart Center Lübeck, Lübeck, Germany; Kjell A. Rein, MD; Oslo University Hospital, Rikshospitalet, Oslo, Norway; Axel Unbehaun, MD; German Heart Institute Berlin, Berlin, Germany; Christoph Klein, MD; German Heart Institute Berlin, Berlin, Germany; Matteo Pozzi, MD; Department of Cardiac Surgery, Hôpital Louis Pradel, Lyon, France; Michele Flagiello, MD; Department of Cardiac Surgery, Hôpital Louis Pradel, Lyon, France; Simon Redwood, MD; St. Thomas’ Hospital, London, UK; Neil S. Kleiman, MD; Department of Cardiovascular Medicine, Houston Methodist DeBakey Heart and Vascular Center, Houston, TX, USA; Mark Peterson, MD, PhD; St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada; Geraldine Ong, MD; St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada; Djeven Deva, MD; St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada; Markus Mach, MD, PhD; Department of Cardiac Surgery, Medical University of Vienna, Vienna, Austria; Tillmann Kerbel, MD; Department of Cardiac Surgery, Medical University of Vienna, Vienna, Austria; Sara Hungerford, MD; St. Vincent’s Hospital, Sydney, NSW, Australia; Francesco Maisano, MD; Ospedale San Raffaele, Milan, Italy; Michaela Hell, MD; Heart Valve Center, Universitätsmedizin Mainz, Mainz, Germany; Jaqueline Da Rocha e Silva, MD; Heart Valve Center, Universitätsmedizin Mainz, Mainz, Germany; Lionel Leroux, MD; Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France; Tanja K. Rudolph, MD; Department of General and Interventional Cardiology, Heart and Diabetes Center North Rhine-Westphalia, Bad Oeynhausen, Germany and Ruhr University Bochum, Bochum, Germany; Kai Friedrichs, MD; Department of General and Interventional Cardiology, Heart and Diabetes Center North Rhine-Westphalia, Bad Oeynhausen, Germany and Ruhr University Bochum, Bochum, Germany; Pierre Berthoumieu, MD; Cardiac Surgery Department, Clinique Pasteur, Toulouse, France; Alberto Pozzoli, MD; CardioCentro Lugano, Lugano, Switzerland; Michael J. Reardon, MD; Department of Cardiothoracic Surgery, Houston Methodist DeBakey Heart and Vascular Center, Houston, TX, USA
Acknowledgements
We want to thank all the CHOICE-MI investigators for their invaluable support to this registry. A full list of CHOICE-MI investigators is given in the Appendix.
Funding
This study was supported by agrant from the German Heart Foundation (DHS).
Conflict of interest statement
S.Ludwig has received travel compensation from Edwards Lifesciences; honoraria from Bayer and Abbott; and was supported by aresearch grant from the German Heart Foundation (DHS). W.Ben Ali has received research grants from Medtronic and Edwards Lifesciences. A.Coisne is proctor for Abbott Vascular; and speaker for Abbott Vascular and GE Healthcare. A.Duncan is aconsultant for and has received honoraria from Abbott, Edwards Lifesciences, and Medtronic. D.Kalbacher has received speaker honoraria from Abbott Medical and Edwards Lifesciences; travel expenses from Abbott Medical and Edwards Lifesciences; and proctor fees from Edwards Lifesciences. T.Rudolph has received speaker honoraria from Abbott Vascular. J.Hausleiter has received consulting fees, speaker honoraria, and support of research projects paid to the institution from Abbott Vascular and Edwards Lifesciences. H.Ruge is amember of the advisory board of Abbott; and aphysician proctor for Abbott and Edwards Lifesciences. M.Adam has received personal fees from Edwards Lifesciences and Boston Scientific; and grants and personal fees from Medtronic. N.Dumonteil has received proctoring and consultancy fees from Abbott Vascular, Boston Scientific, Edwards Lifesciences, and Medtronic. D.Tchétché has received proctoring and consultancy fees from Abbott Vascular, Boston Scientific, Edwards Lifesciences, and Medtronic. M.Adamo has received speaker fees from Abbott and Medtronic. G.Dahle is aproctor and speaker for Abbott and aspeaker for Edwards Lifesciences. M.Taramasso is aconsultant for Abbott, Edwards Lifesciences, Boston Scientific, Shenqi Medical, Simulands, Occlufit, MTEx, MEDIRA, 4tech, and CoreMedic; and has received fees from Cardiovalve. J.Kempfert has received speaker honoraria from Edwards Lifesciences, Medtronic, Abbott, and CryoLife. G.H.L.Tang is aphysician proctor and consultant for Medtronic, consultant and TAVR physician advisory board member for Abbott Structural Heart, consultant for NeoChord, and advisory board member for JenaValve. S.Goel is aconsultant for Medtronic and part of the speakers’ bureau of Abbott Structural Heart. N.Fam is aconsultant for Edwards Lifesciences, Abbott, and Cardiovalve. M.Andreas is aproctor, consultant and speaker for Edwards Lifesciences, Abbott, Medtronic, Boston, and Zoll; and has received institutional research grants from Edwards Lifesciences, Abbott, Medtronic, and LSI.R.S.von Bardeleben has received consulting and speaker honoraria from Abbott Vascular, Edwards Lifesciences, and Medtronic; as well as research project grants paid to the university from Abbott Vascular and Edwards Lifesciences. L.Conradi is advisory board member for Abbott, Medtronic, and Boston Scientific; and has received personal fees from Edwards Lifesciences. The other authors/collaborators have no conflicts of interest to declare, regarding this manuscript.
Supplementary data
To read the full content of this article, please download the PDF.

