Abstract
Aims: Transcatheter mitral valve-in-valve implantation (TMViVI) for the treatment of failing mitral xenografts or recurrent mitral regurgitation after surgical ring implantation is an emerging therapy for patients in need of repeated mitral valve surgery. Despite the fact that these procedures have been shown to be feasible and effective, haemodynamic data after TMViVI are still limited in the literature.
Methods and results: Twelve patients (logES: 39.2±23.5%) were treated either by transapical (n=7) or transseptal (n=5) TMViVI, as a valve-in-valve (ViV, n=8) or valve-in-ring (ViR, n=4) implantation. Left atrial pressures (LAP), transmitral gradients and right heart haemodynamics (Swan-Ganz catheterisation) were studied before and after TMViVI. Procedural success was 100%, mitral regurgitation after TMViVI was mild in one, trace in five and absent in six patients. Thirty-day mortality was 0%. Left atrial pressures decreased significantly after valve implantation (before LAPmean/v-wave: 24.3/44.1 mmHg; after LAP/v-wave 15.9/22.1 mmHg; p<0.001) and cardiac output increased significantly. Transmitral gradients corresponded to mitral surface areas between 1.7 and 3.5 cm2, and were thus very acceptable in terms of the high surgical risk population.
Conclusions: In conclusion, TMViVI with the balloon-expandable SAPIEN XT valve for ViV or ViR implantation is feasible with promising acute transmitral haemodynamic data. Nevertheless, sustained long-term performance remains to be demonstrated in the future.
Introduction
Surgical valve replacement (SVR) has produced excellent results over recent decades and biologic xenografts as opposed to mechanical valves are increasingly preferred for aortic, mitral and tricuspid valve replacement1,2. Nevertheless, due to an increase in the life expectancy of patients, redo valve surgery is increasingly necessary and, due to technical aspects3 or poor clinical condition4, associated with increased operative mortalities. Extensive coverage by neoendocardium or calcification is frequently observed, making reoperation sometimes extremely difficult. Thus, irreparable damage, one of the greatest nightmares of a cardiac surgeon, may occur3. In addition, the remaining annulus can be very weak and paravalvular leaks frequently ensue5. Therefore, the avoidance of the removal of the malfunctioning bioprosthesis would decrease significantly the surgical burden of these patients. In this regard, excising only the leaflets of the damaged bioprosthesis and leaving in situ the old ring on which the mechanical valve was sutured was already being proposed by surgeons roughly 20 years ago3,6.
After the successful introduction of transcatheter aortic valve implantation (TAVI) in 20027, the valve-in-valve (ViV) concept as a treatment for failing biological aortic prostheses8,9 and mitral prostheses10-17 gained increasing attention. In addition, balloon-expandable valves have been implanted successfully but only occasionally into mitral annuloplasty rings18,19. Until now only sparse data about the haemodynamic properties of SAPIEN valves in the mitral position have been available.
The present study reports our experience with the current CE-marked Edwards SAPIEN/SAPIEN XT valves after implantation in the mitral position – either in degenerated xenografts or failing surgical annuloplasty rings – with special focus on clinical outcomes and the invasive haemodynamics after implantation.
Methods
STUDY DESIGN AND PATIENT POPULATION
Between November 2010 and August 2012, a total of twelve high-risk patients with either severe degeneration of a mitral bioprosthesis or recurrent mitral regurgitation after mitral ring annuloplasty underwent a transcatheter mitral valve-in-valve implantation (TMViVI) or transcatheter mitral valve-in-ring implantation (TMViRI) at our institution. Four patients had a failing Carpentier-Edwards (CE) S.A.V. xenograft (Edwards Lifesciences, Irvine, CA, USA), two patients had a malfunctioning Medtronic Hancock II prosthesis (Medtronic, Minneapolis, MN, USA), one patient had a stenosed Carpentier-Edwards Perimount Magna (Edwards Lifesciences), one patient had a mixed degenerated St. Jude Medical Epic (St. Jude Medical, St. Paul, MN, USA) and four patients were formerly treated with annuloplasty rings (Medtronic Duran AnCore ring [n=1], Edwards Physio ring I [n=2] and II [n=1]) (Table 1 and Table 2). Having obtained informed consent, TMViVI or TMViRI was performed under general anaesthesia in eight patients due to concomitant use of transoesophageal echocardiography (TEE). In four patients only analgosedation was chosen, since TEE had to be omitted due to severe pulmonary disease making oral intubation and pulmonary ventilation undesirable.
DEVICE DESCRIPTION AND PROCEDURE
The balloon-expandable Edwards SAPIEN® or the SAPIEN XT® bioprosthesis (Edwards Lifesciences) was used in this study. All patients received acetylsalicylic acid 100 mg, which was started before the procedure and continued indefinitely. A 600 mg loading dose of clopidogrel was administered the day before the procedure, followed by 75 mg daily for one month. Standard antibiotic prophylaxis with intravenous cefazoline was started before the procedure and continued for three to five days. During the intervention, 100 IU/kg of heparin was administered to achieve an activated clotting time of 250-300 seconds. All operations were performed in a hybrid operating room. The retrograde transapical (TA) approach (n=7 standard technique20) and the antegrade transseptal (TS) approach (n=4 transfemoral, n=1 transjugular) were used, as previously published by our group15-17. Preoperative measurement of the internal diameter of the mitral bioprosthesis was conducted in most patients by bench-testing before treatment. In this regard various sizes of SAPIEN valves were implanted in a “dry run” inside the prosthetical valve or ring to achieve the minimal oversizing needed for prevention of valve embolisation. Moreover, multislice computed tomography (MSCT) and transoesophageal echocardiography (TEE) measurements were performed in most patients. Balloon valvuloplasty before valve implantation was performed only in a single patient (for sizing purposes). This particular patient was treated by a 23 mm SAPIEN XT valve fitting sufficiently into a 27 mm Carpentier-Edwards (SAV) prosthesis. Valve implantations were basically guided by fluoroscopy, and no contrast angiography was necessary during implantation. Once the SAPIEN valve was deployed, ventriculography and transoesophageal echocardiography in addition to invasive haemodynamic recordings (transvalvular gradient, left ventricular end-systolic/end-diastolic pressure, left atrial pressure, systemic arterial and pulmonary arterial pressure, pulmonary capillary wedge pressure and cardiac output) were taken. Post-ballooning was done if echocardiography revealed persistent paravalvular regurgitation. Immediately after the procedure, the temporary pacemaker (PM) was removed and the patients were extubated if possible.
DATA COLLECTION AND DEFINITIONS
All clinically relevant baseline and follow-up variables were recorded and entered into a database. Technical success was defined as stable device placement and function as assessed by angiography and echocardiography. Device success was defined as success according to VARC21. Invasive haemodynamic data were obtained immediately before and after valve implantation. In addition, oxymetry runs were performed before and after transseptal transcatheter mitral valve implantation (TMVI) to assess/exclude possible interatrial shunting. Post-procedural regurgitation was acutely assessed by left ventricular angiography, transoesophageal echocardiography and by transthoracic echocardiography during the follow-up visits at six weeks and six months, respectively.
STATISTICAL ANALYSIS
Continuous data were described as means and standard error of the mean (SEM). Differences before and after TMVI were analysed with paired t-tests. Categorical data were described with absolute and relative frequencies. Differences between categorical variables were evaluated with the chi-square test or with Fisher’s exact test in case of small expected cell frequencies. All p-values are two-sided. For overall tests p≤0.05 was considered significant. All calculations were performed with Prism for Windows, version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA) and Microsoft Excel for Mac 2011, version 14.0.0 (Microsoft, Redmond, WA, USA).
Results
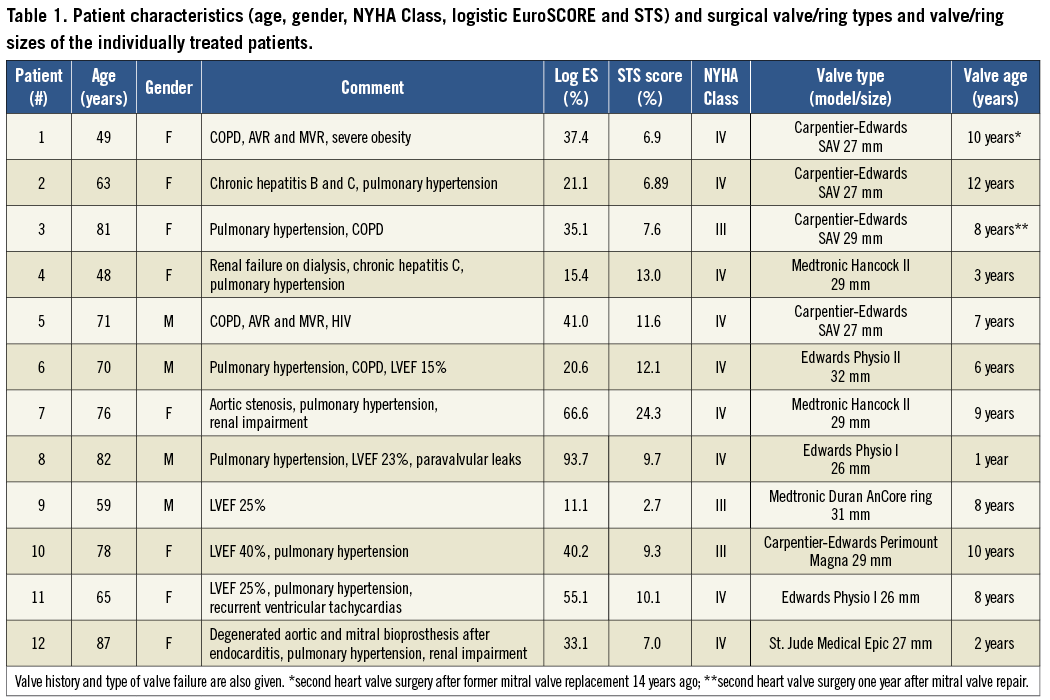
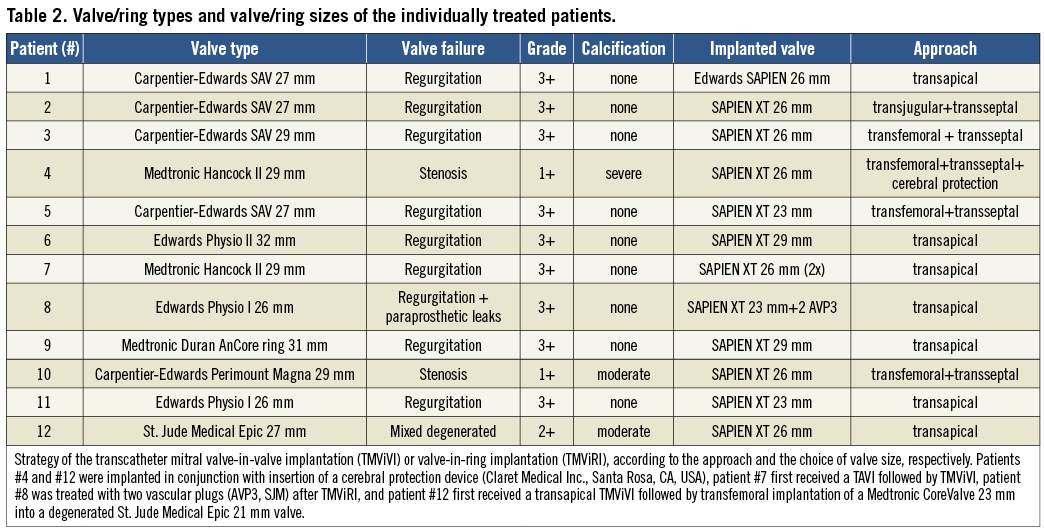
Overall baseline characteristics of the patients are summarised in Table 1. The mean logistic EuroSCORE was 39.1±23.5% (range: 11.1-93.7), and 100% of the patients were in New York Heart Association functional Class III or IV. The mechanism of valvular dysfunction was severe mitral regurgitation in nine patients, stenosis in two patients and mixed degeneration in one patient. Seven out of twelve patients were in atrial fibrillation and five patients had more than one open heart surgery in their past medical history. The mean transvalvular pressure gradient was 7.9±4.4 mmHg and the preprocedural mean calculated valve area was 1.61±0.5 cm2 (Table 2). Two patients with a degenerated mitral bioprosthesis had concomitant severe aortic stenosis (patient #7) or a stenotic aortic bioprosthesis (patient #12, with a 21 mm St. Jude Medical Epic valve) requiring contemporaneous transcatheter aortic valve implantation and TMViVI (Figure 1 and Figure 2) into the failing mitral bioprosthesis (both 26 mm SAPIEN valves). Four patients were treated by transapical SAPIEN implantation for recurrent mitral regurgitation after mitral repair with a Medtronic Duran AnCore 31 mm ring, a Physio II 32 mm annuloplasty ring (Figure 3), and two patients with a Physio I 26 mm ring, respectively. One patient with a Physio I 26 mm ring additionally displayed leakages next to the sutured ring (due to avulsion of the sutures), which were successfully treated by implantation of two vascular plugs (AVP3; St. Jude Medical GmbH, Eschborn, Germany) (Figure 4).
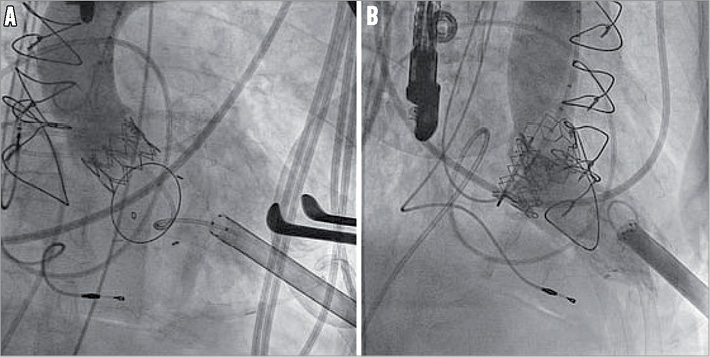
Figure 1. Transapical transcatheter mitral valve-in-valve implantation (TMViVI) immediately after transapical transcatheter aortic valve implantation (TAVI).
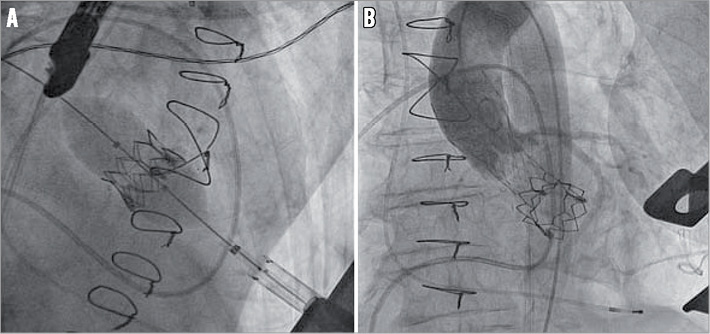
Figure 2. Transapical transcatheter mitral valve-in-valve implantation (TMViVI) with an Edwards SAPIEN XT followed immediately by transfemoral aortic ViV implantation with a Medtronic CoreValve.
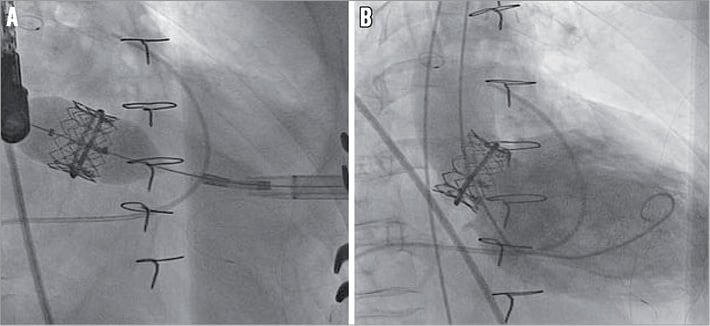
Figure 3. Transapical transcatheter mitral valve-in-ring implantation (TMViRI).
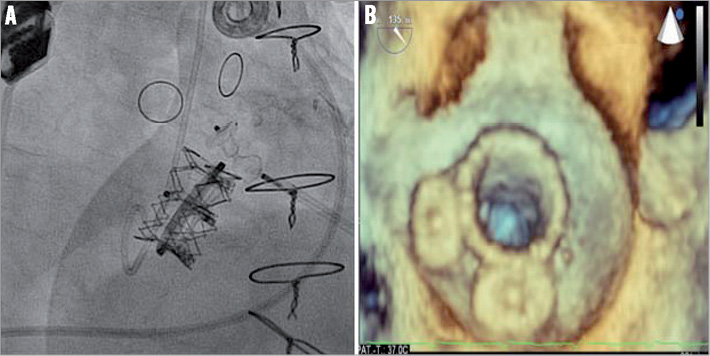
Figure 4. Transapical closure of two para-annular leaks in conjunction with transapical valve-in-ring implantation with a 23 mm SAPIEN XT prosthesis into a 26 mm Edwards Physio I ring (A). 3D TEE surgical view after transapical transcatheter mitral valve-in-ring implantation (TMViRI) and two vascular plugs (AVP3) placed into leaks next to the sutured ring (avulsion of the sutures, B).
ACUTE PROCEDURAL SUCCESS
Acute technical success was achieved in all patients and conversion to open heart surgery was not performed in any case (SAPIEN-TS, n=5; SAPIEN-TA, n=7). Post-procedural intravalvular regurgitation was not seen in any patient. A significant paravalvular leak immediately after valve implantation was present in six patients, thus post-ballooning was done in these six patients. Post-procedural paravalvular regurgitation as a final result was graded as trace in five patients (CE SAV 27 mm treated by SAPIEN-TA 26 mm, n=1; CE SAV 27 mm treated by SAPIEN-TS 23 mm, n=1; CE Perimount Magna 29 mm treated by SAPIEN-TS 26 mm, n=1; Medtronic Duran AnCore 31 mm ring treated by SAPIEN-TA 29 mm, n=1; Physio II 32 mm ring treated by SAPIEN-TA 29 mm, n=1) and mild in one patient, respectively. In fact, the mild regurgitation was not related to the procedure, since the regurgitation was next to the down-sized annuloplasty ring (Physio I 26 mm, see above).
There was no intraprocedural or 30-day mortality. No apical haemorrhage or vascular bleeding was encountered, and no reoperation for bleeding or tamponade was required. In one case haemodynamic instability was encountered before the procedure and support with cardiopulmonary bypass was transiently implemented during valve implantation. Haemodynamic and valvular function was very satisfactory in all patients (Table 3, Figure 5 and Figure 6). The mean postprocedural transmitral gradient was 5.3±2.3 mmHg. No patient displayed obstruction of the left ventricular outflow tract or significant interatrial shunting (after transseptal TMVI, n=5). A major stroke within 48 hours after TA TMViVI was observed in one patient. Vascular access complications with the transseptal approach occurred in n=0 patients (VARC minor 0%, VARC major 0%). All patients were extubated (if intubated) right after the procedure. By contrast, one patient treated by TA TMViVI had to be kept intubated for several days due to continuous diffusion capacity problems. In addition, this patient had to be treated by multiple wound revisions at the thoracic surgical site and developed acute kidney failure that had to be treated by haemodialysis.
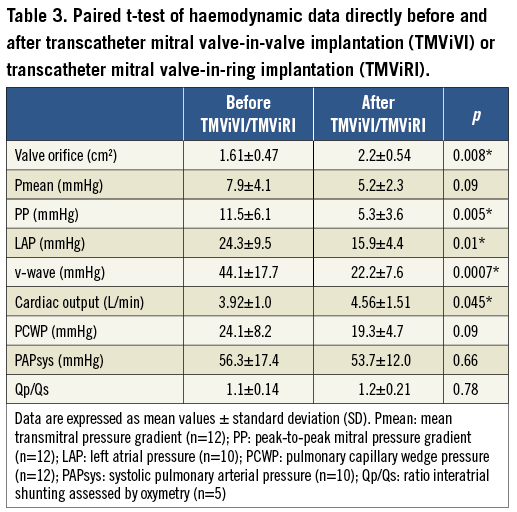
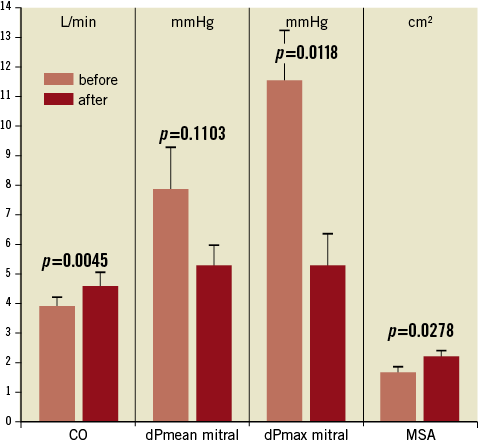
Figure 5. Transmitral invasive haemodynamics with cardiac output (CO), mean transmitral pressure gradient (dPmean mitral), maximal transmitral pressure gradient (dPmax mitral) and mitral surface area (MSA) before and after treatment. Pooled data of 12 patients with n=8 patients suffering from a degenerated xenograft and n=4 patients with recurrent MR after mitral repair, treated by TMViVI or TMViRI, respectively. Data are combined irrespective if implanted transfemorally or transapically.
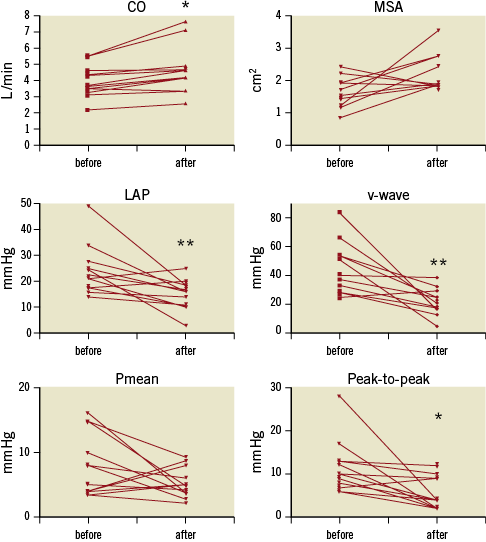
Figure 6. Individual transmitral invasive haemodynamics with cardiac output (CO), mitral surface area (MSA), left atrial pressure (LAP), v-wave, mean transmitral pressure gradient (dPmean mitral), and maximal transmitral pressure gradient (dPmax mitral) before and after treatment. Pooled data of eight patients suffering from a degenerated xenograft and n=4 patients with recurrent MR after mitral repair, treated by TMViVI or TMViRI, respectively. Data are combined irrespective if implanted transfemorally or transapically.
FOLLOW-UP CLINICAL RESULTS
Overall mortality at 30 days was 0%. At 30 days, the mean NYHA functional class declined from 100% in NYHA Class III or IV (preprocedural) to 58.3% in Class I or II at six weeks (see Tables; p<0.01). All but one patient were clinically improved according to NYHA class six weeks after the procedure (Figure 7 and Figure 8). No other device-related adverse events were observed. Mean transvalvular pressure gradients remained in the range of the invasive measurements immediately after valve implantation. Unfortunately, two patients (patients #8 and #11) died after 92 days and 105 days, respectively. Patient #8 died due to multiple organ dysfunction syndrome, whereas patient #11 died due to incessant ventricular tachycardia (VT), despite initial successful VT ablation. At the six-month follow-up all surviving patients displayed stable device function (mean gradient 6.1±2.2 mmHg, mitral surface area 2.0±0.3 cm2) without significant mitral regurgitation. There was no new paravalvular leakage observed during follow-up. No atrial clots were detected on a routine follow-up echocardiogram at six months.
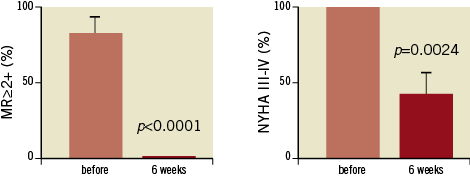
Figure 7. Mitral regurgitation (MR) (A) and functional class according to NYHA (B) before and six weeks after treatment. Pooled data of 12 patients with n=8 patients suffering from a degenerated xenograft and n=4 patients with recurrent MR after mitral repair, treated by TMViVI or TMViRI.
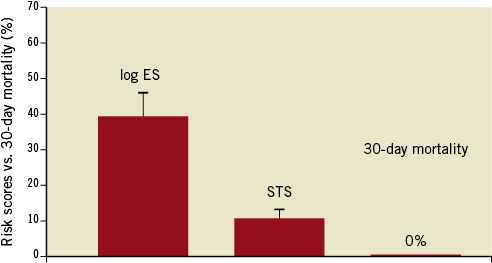
Figure 8. 30-day mortality compared to the average risk scores (logistic EuroSCORE and STS) after TMViVI and TMViRI.
Discussion
The major findings of the present paper are:
– Transcatheter valve implantation with balloon-expandable valves into failing surgical mitral xenografts can be performed with high procedural success rates using either a transseptal or a transapical approach.
– Invasive haemodynamics revealed very acceptable results with an increase in cardiac output and mitral surface areas between 1.7 and 3.5 cm2.
– Patient outcome was very satisfactory with no mortalities within 30 days and a marked clinical improvement during the follow-up.
In the past, the feasibility of transcatheter mitral valve-in-valve implantation (TMViV) providing significant clinical improvement has been demonstrated repeatedly. Nevertheless, the available devices are only approved for treatment of aortic valvular stenosis in high surgical risk patients and experience concerning the treatment of failing xenografts is limited22. With regard to failing xenografts in the mitral position, mainly transapical implantations of SAPIEN valves have been reported in the literature10. Interestingly, the valve-in-valve concept in the mitral position was first demonstrated in a sheep model by Walther and colleagues13, and the authors proposed a transatrial approach to the mitral valve. The largest updated series of valve-in-valve implantations for failing surgical mitral xenografts23 so far comprised eleven patients (initially with seven patients24). Using either a transseptal approach or a direct transatrial approach in the first two patients, both attempts failed because of the inability to align the valve coaxially within the prosthetic valve. The first patient died within 24 hours after conversion to open heart surgery and the second patient, subsequently treated transapically, died on day 45. As a result, all subsequent implantations were performed transapically with excellent outcomes. In their series, TMViVI was associated with a reduction in mean gradient from 12.9 to 8.0 mmHg and an increase in area from 0.7 to 1.7 cm2. Their observations matched our findings nicely with an average mitral orifice area of 2.2±0.5 cm2. In addition, similar to the observation by Webb and colleagues, we found a significant decrease in peak-to-peak transmitral gradients, although the mean transmitral gradient remained essentially unchanged23,24. Very recently, Cerillo and colleagues12 reported on a series of three patients with failing mitral bioprostheses treated by TA TMViVI. Due to migration of the SAPIEN valve into the left ventricular outflow tract, the first patient had to be converted to open heart surgery due to severe subaortic obstruction, but died of multi-organ failure within 24 hours. It is our understanding that it is of decisive importance to prevent the implantation of a too oversized valve into the rigid ring of a surgical bioprosthesis. An underexpanded SAPIEN valve within a small surgical prosthesis will definitely function suboptimally with an increased transvalvular gradient, impaired leaflet coaptation, reduced durability, or may even embolise during implantation. As frequently hypothesised by surgeons, we did not see any significant pressure gradient across the aortic valve or along the left ventricular outflow tract after TMViVI in any patient, again demonstrating the feasibility of TMViVI.
Despite the suboptimal initial results with TMViVI (in-hospital mortality 28.6%24 and 33.3%12), TA TMViVI has been repeatedly proposed to offer a safer approach for high-risk redo surgical patients. Indeed, within the updated largest published series of 11 patients by Webb and co-workers23, all patients were successfully treated by TA TMViVI, with no 30-day mortality. The improved results have been related to the fact that less frail patients were treated in the subsequent series12,23. This assumption may be supported by the fact that the initial procedure-related fatalities occurred in patients with rather high logistic EuroSCOREs (31.2% and 37.3%24, as well as 81.5%12). This is in line with the observation that a logistic EuroSCORE >30% has been reported to be the single most important predictor of death after TA TAVI25. Nevertheless, the cases presented in our study involved patients with multiple comorbidities (age: 49-87 years; logistic EuroSCORE: 11.1%-93.7%). All patients were at increased surgical risk for operation as evidenced by the STS score (10.0±5.3%) since all patients had a previous cardiac surgery, heart failure NYHA Class III to IV, and most had severe pulmonary hypertension (8 out of 12 patients). Two patients suffered from liver cirrhosis related to chronic hepatitis C infection. Despite these rather sick patients, we did not observe a single fatality, irrespective of the fact that eight out of twelve patients had a logistic EuroSCORE >30%.
By contrast to the recommended transapical technique for TMViVI, we decided to use an antegrade transseptal approach in a subset of patients. The reasons for rejecting the transapical approach in our series were: 1) anatomical considerations such as very large mammaries or excessive scarring of the skin after the previously performed sternotomy; 2) clinical considerations, i.e., we did not want to intubate the patient due to severe pulmonary lung disease; and 3) the patient’s wish. Other reasons might be a higher enzyme leak and/or periprocedural myocardial infarction, higher apical akinesis, or distal LAD occlusion/VSD for apical access.
Moreover, in a quick search of the literature, at least 12 cases were found with a false left ventricular apical aneurysm as a late complication after TA TAVI12,26,27. Thus, due to these unfavourable observations and the clinical need for being less invasive, we were encouraged to use the transseptal approach in five out of twelve patients.
The only advantage with the transapical approach is its direct access to the failing mitral bioprothesis (short distance and immediate steering possibilities). A disadvantage is the fact that the bioprosthesis needs to be crossed in a retrograde fashion, possibly leading to more extensive damage of the surgical bioprosthesis with a higher likelihood of a haemodynamic compromise (as was seen in two TA TMViVI). Nevertheless, crossing the septum during TS TMViVI might be a hurdle (especially if performed via the transjugular route)16. In this regard, the establishment of an arteriovenous wire loop allowing for push and pull manipulations turned out to be very helpful. Lately, successful TS TMViRI has been reported by others19. This might be especially important in patients with severe reduced left ventricle (LV) function or other hostile conditions at the LV apex.
In general, it is of major importance to be familiar with the shape of surgical implanted valves prior to performing a TMViV. Nevertheless, the implantation is usually facilitated by the visibility of the bioprosthetic ring or leaflet attachments, serving as an orientation tool (if radiopaque). In addition, almost complete abolition of mitral regurgitation with very acceptable transvalvular gradients can be achieved. The latter is largely explained by the very low profile of these catheter heart valves, if proper size matching of the inner diameter of the xenograft to the SAPIEN valve is performed, thereby preventing central leakage or significant intravalvular obstruction with high residual gradients. In fact, we were extremely eager to obtain full expansion of the SAPIEN valve (two patients received a relatively small valve, i.e., a 23 mm SAPIEN into a 27 mm Carpentier-Edwards SAV prosthesis and a 26 mm SAPIEN XT for a CE Perimount Magna 29 mm). This largely explains the rather low transmitral gradients in our series.
Summary
Here we report our overall experience in twelve patients with the use of a balloon-expandable valve for treatment of degenerated mitral xenograft or recurrent regurgitation after ring annuloplasty in the mitral position. Procedural success (adapted to VARC, although not designed for mitral interventions) was independent if the chosen approach was transapical or transseptal, and no structural deterioration was demonstrated during the relatively short follow-up. Most of the patients had a dramatic improvement in their symptoms and remained in NYHA Class I/II at the latest follow-up.
Limitations
This is an observational study of only a small group of patients with three different approaches for TMViVI. Invasive haemodynamic measurements can carry numerous pitfalls during acute structural intervention (use of general anaesthaesia, possible left or right atrial shunting, unstable haemodynamic state). Despite these limitations, we found only minor differences related to echocardiographic data and we disclosed significant shunting with the use of oxymetry.
Conclusions
In general, all cases described here, together with previous reports, demonstrate the feasibility of TMViVI for treatment of a degenerated mitral bioprosthesis or recurrent mitral regurgitation after surgical ring implantation using a balloon-expandable valve. After TMViVI, there was almost complete resolution of mitral regurgitation, and the vast majority of patients had a satisfactory clinical outcome at six months. Hence, valve-in-valve or valve-in-ring implantation might become a valuable therapy option for many elderly patients in the future. However, the choice of valves and rings during the index surgical treatment remains decisive for this elegant treatment option in the future.
Conflict of interest statement
U. Schäfer is a clinical proctor for Edwards Lifesciences Inc. and KH Kuck has received honoraria payments from Edwards Lifesciences Inc. The other authors have no conflicts of interest to declare.

