Abstract
There is a growing practice of transcatheter treatment of failed mitral valves after cardiac surgery, including valve-in-valve and valve-in-ring. Although commonly successful, these procedures can be associated with device malposition (including delayed malposition) and elevated post-procedural gradients (especially when performed inside small surgical valves). Valve-in-ring procedures have elevated risks of left ventricular outflow tract obstruction and post-procedural regurgitation. Careful patient selection and meticulous evaluation of patient anatomy and surgical implant characteristics are essential to achieve optimal clinical results with mitral valve-in-valve and valve-in-ring implantation.
Introduction
Bioprosthetic surgical valves and rings are commonly implanted during mitral valve surgical interventions. Degeneration of bioprosthetic leaflets can result in valve stenosis and/or regurgitation. Stenosis and regurgitation can also follow mitral ring implantation. Treatment of patients with failed mitral valves after cardiac surgery is a clinical challenge as these patients are frequently elderly, frail, and have ventricular dysfunction. While surgery is considered the standard of care, reoperation carries significant morbidity and mortality risk.
Previous reports have demonstrated the feasibility of treating degenerated mitral bioprostheses and mitral valve rings with transcatheter heart valves (i.e., valve-in-valve [ViV] and valve-in-ring [ViR])1,2. Preliminary data from the Valve-in-Valve International Data (VIVID) Registry reveal that, although procedural success is achieved in the majority of these cases, there are several safety and efficacy concerns. The following review will describe some of the main considerations in mitral ViV and mitral ViR procedures.
The characteristics of the failed surgical device
A critical step prior to a mitral ViV or ViR procedure is to understand the characteristics of the previously implanted device. Each surgical device has unique geometrical characteristics beyond, and not directly related to, label size. These unique characteristics include variations in internal diameter, length, the optimal target for implantation, and fluoroscopic characteristics, amongst many others. Many of these parameters can be retrieved from manufacturer’s publications and websites or from a freely downloadable ViV app3.
Similarly, for a mitral ViR procedure fundamental knowledge would be an understanding of the specific ring, including its flexibility, three-dimensional geometry, and dimensions. In general, a complete ring will provide better fixation for transcatheter valve implantation than an incomplete ring. Additionally, a flexible ring is more favourable than a rigid ring, as it will conform better to implantation of a circular transcatheter valve, with better paravalvular sealing as well as superior leaflet function and durability.
Procedural access
Transcatheter access for mitral valve implantation can be open surgical or a fully percutaneous approach. Open surgical access generally incorporates a thoracotomy, most commonly combined with a transapical approach and much less commonly with a direct left atrial approach. Fully percutaneous access can be achieved via the femoral or less commonly the jugular vein, combined with transseptal puncture. Data from the VIVID Registry reveal that the transseptal approach was utilised in only 16% of ViV cases and 28% of ViR cases. However, there has been a dramatic growth in the less invasive transseptal approach - from 15% in 2013, increasing to 25.4% of procedures from 2014 to early 2016.
The transapical and transseptal valve procedures differ in many respects (Figure 1, Moving image 1, Moving image 2). The cardiac apex is substantially closer to the mitral valve than the peripheral veins. As a result, transapical implantation may enable better control over the implant position. However, the transseptal approach is less invasive, eliminating the need for a thoracotomy, reducing the risk of trauma to the left ventricle. Data from the VIVID Registry show an improvement in myocardial contraction in patients with left ventricular dysfunction treated by transseptal approach that was more pronounced than in those treated transapically4. We can speculate that in the future the majority of transcatheter mitral valve implantations will be performed via transseptal approach, while transapical access will be reserved for selected cases5.
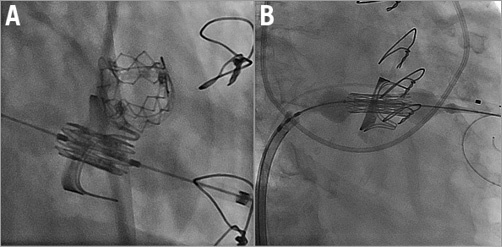
Figure 1. Mitral valve-in-valve implantation. A) Transapical access. B) Transseptal access.
Transcatheter devices used in mitral ViV/ViR
In the last three years, a growing number of dedicated devices have been implanted inside native mitral valves. These devices were very rarely used to treat failed bioprosthetic valves or failed valves after ring implantation. Devices implanted in failed mitral valves and rings have almost always been the same transcatheter valves implanted inside native aortic valves. According to the VIVID Registry the most common devices used in mitral ViV/ViR are SAPIEN devices: SAPIEN, SAPIEN XT and SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA), accounting for approximately 90% of cases. However, other balloon-expandable transcatheter valve devices were also used, including Melody® (Medtronic, Minneapolis, MN, USA) and Inovare® (Braile Biomedica, São José do Rio Preto, Brazil) valves. The last few years have seen interest in retrievable devices, the Lotus™ (Boston Scientific, Marlborough, MA, USA) and Direct Flow® valves (Direct Flow Medical, Santa Rosa, CA, USA), which require transapical access for mitral implantation. A retrievable device could be advantageous especially in cases at risk for device malposition, post-procedural regurgitation or left ventricular outflow tract (LVOT) obstruction.
Procedural and delayed device malposition
Device malposition occurred in approximately 7% of mitral cases in the VIVID Registry. In the majority of these cases a second valve was required. Surgical valves with poor fluoroscopic markers, such as Mosaic® (Medtronic) and Epic™ (St. Jude Medical, St. Paul, MN, USA) may increase the risk for device malposition.
A unique phenomenon in procedures performed in the mitral position is delayed mitral malposition. In these cases, a device was accurately positioned during the index procedure but dislocated towards the left atrium, usually a couple of weeks after implantation (Figure 2, Moving image 3-Moving image 5). This complication is commonly related to inadequate transcatheter device oversizing. It seems that in mitral ViV/ViR procedures the amount of oversizing should be greater than in the aortic position in order to prevent delayed malposition6. This complication could be easily diagnosed by a simple transthoracic echocardiogram and even by a lateral chest X-ray (Figure 2).
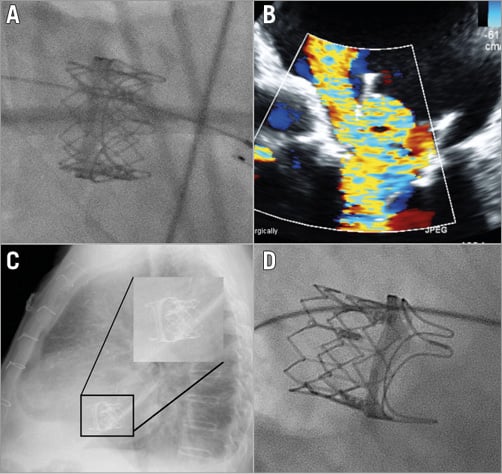
Figure 2. Delayed malposition after mitral valve-in-valve implantation. A) A 26 mm SAPIEN XT was implanted in a PERIMOUNT #27 (Edwards Lifesciences). B) Six weeks after implantation the patient represented with heart failure due to severe mitral regurgitation. Malposition was documented with a lateral chest X-ray (C) and fluoroscopy (D). The patient underwent a successful repeat ViV procedure using a 29 mm SAPIEN XT.
Left ventricular outflow tract obstruction
Flow obstruction in the LVOT after mitral ViV/ViR can be a life-threatening complication (Figure 3, Moving image 6, Moving image 7). According to the VIVID Registry, this risk (defined as clinically evident LVOT obstruction) is much higher after ViR implantation than it is in those performed inside a calcified mitral annulus7. The risk for LVOT obstruction should be thoroughly assessed in all candidates for transcatheter mitral procedures and is related to different characteristics: anatomical (e.g., the angulation between the aortic and mitral planes, septal thickness), mitral valve leaflets (e.g., pericardial leaflets, calcified leaflets, long leaflets), and procedural characteristics (e.g., depth of implantation - more to the ventricle, using a non-retrievable transcatheter device). Cases at risk for LVOT obstruction could be identified by simple transthoracic echocardiographic views that may reveal the relationship between the mitral and aortic planes, i.e., parasternal long-axis and apical five-chamber views. Cardiac CT is a very useful tool that may further delineate the anatomy of the LVOT before and after a simulated transcatheter heart valve is implanted8. Following mitral valve implantation, a neo-LVOT is created, a complex structure that is also dynamic throughout the cardiac cycle. Its size may support a decision related to the risk of clinical LVOT obstruction following implantation.
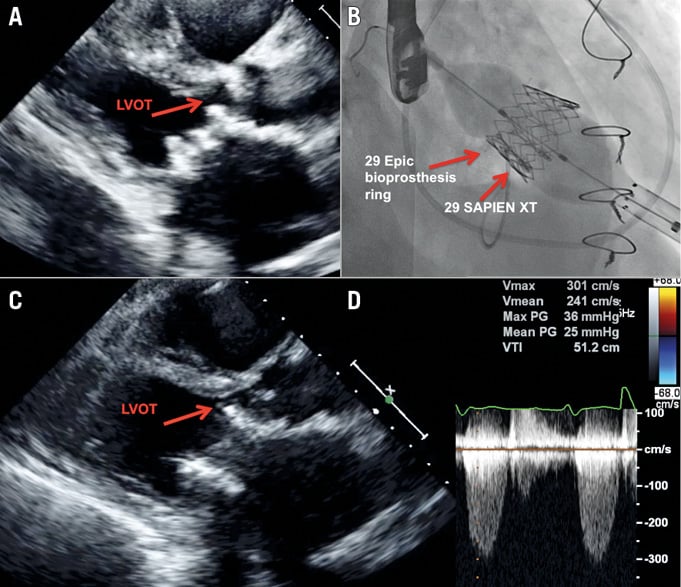
Figure 3. Left ventricular outflow tract obstruction following mitral valve-in-valve. A) Transthoracic echocardiography (parasternal long-axis view) revealing an acute angle between the aortic and mitral valve planes with septal hypertrophy with and only mild residual LVOT space. B) Mitral valve in valve with 29 mm SAPIEN XT implanted inside 29 mm label size Epic. C) Post implantation echocardiographic exam reveals only minimal residual LVOT space. D) Elevated gradients across the LVOT after mitral valve-in-valve.
Elevated post-procedural gradients
In general, surgically implanted mitral valves are considerably larger than surgical aortic valves. Elevated post-procedural gradients that are common in the aortic position are less of a concern after mitral ViV procedures9. Nevertheless, it seems that elevated gradients can be a limitation of mitral ViV procedures as well. According to MVARC, a mean transmitral gradient of 5 mmHg or greater should be considered device failure10. Even when using a much less stringent definition (mean gradient 10 mmHg or greater), elevated post-procedural gradients in small surgical mitral valves were reported in 27% of cases in the VIVID Registry. The clinical significance of these suboptimal haemodynamic results and potential effect on device durability need to be explored further.
Post-procedural mitral regurgitation
Regurgitation after ViV procedures is uncommon and occasionally a result of a leakage originating outside the surgical valve ring (surgical valve paravalvular leakage). In the VIVID Registry, significant regurgitation (≥ moderate) occurred in only 2.6% of mitral ViV cases. However, the risk of post-procedural regurgitation is greater in the setting of mitral rings. In the VIVID Registry, significant regurgitation occurred in 15% of ViR cases, with more frequent requirement for post-dilatation or a second transcatheter valve. Post-procedural regurgitation is a significant limitation of ViR procedures, especially those performed in relatively rigid rings.
Conclusion
There is a growing practice of transcatheter treatment of failed mitral valves after cardiac surgery including both ViV and ViR procedures. The most common access in these procedures is transapical but a growing number of cases are being performed using a transseptal approach. The most common devices used in these procedures are balloon-expandable devices; however, transapical implantation of retrievable devices is occasionally used as well. Mitral ViV and ViR procedures are associated with device malposition (including delayed malposition), and elevated post-procedural gradients (especially when performed in small surgical valves). ViR procedures have elevated risks of LVOT obstruction and post-procedural regurgitation. Careful patient selection and meticulous evaluation of patient anatomy and surgical implant characteristics are key in order to achieve optimal clinical results.
Conflict of interest statement
D. Dvir and J. Webb are consultants to Edwards Lifesciences.
Supplementary data
Moving image 1. Transseptal mitral-valve-in-valve implantation.
Moving image 2. Left ventricular injection after mitral valve-in-valve implantation.
Moving image 3. Mitral valve-in-valve using a 26 mm SAPIEN XT inside #27 Perimount.
Moving image 4. Fluoroscopy showing delayed malposition.
Moving image 5. Transoesophageal echocardiogram after delayed malposition.
Moving image 6. Echocardiogram of a failed mitral valve before valve-in-valve attempt.
Moving image 7. Echocardiogram showing left ventricular outflow tract obstruction.
Supplementary data
To read the full content of this article, please download the PDF.
Transseptal mitral-valve-in-valve implantation.
Left ventricular injection after mitral valve-invalve implantation.
Mitral valve-in-valve using a 26 mm SAPIEN XT inside #27 Perimount.
Fluoroscopy showing delayed malposition.
Transoesophageal echocardiogram after delayed malposition.
Echocardiogram of a failed mitral valve before valve-in-valve attempt.
Echocardiogram showing left ventricular outflow tract obstruction

