Abstract
BACKGROUND: Data comparing transcatheter mitral valve implantation (TMVI) with surgical mitral valve replacement (SMVR) are lacking.
AIMS: This study sought to compare the 30-day Valve Academic Research Consortium (VARC)-3 device success of TMVI with that of SMVR.
METHODS: Matching protocol combined exact matching (sex, atrial fibrillation, previous surgical aortic valve replacement [SAVR] or coronary artery bypass grafting [CABG]), coarsened exact matching (age) and propensity score matching (body mass index, mitral valve pathology and concomitant tricuspid regurgitation).
RESULTS: A total of 40 Tendyne TMVI and 80 SMVR patients with similar baseline characteristics were analysed (TMVI vs SMVR): age (78 years [interquartile range [{IQR} 75; 80] vs 78 years [IQR 73; 80]; p=0.8), female (60% vs 60%; p=1.0), atrial fibrillation (67.5% vs 63.7%; p=0.8), previous SAVR (12.5% vs 10.0%; p=0.8), previous CABG (20.0% vs 16.2%; p=0.8), body mass index (25.54 kg/m² vs 25.24 kg/m²; p=0.7) and valve pathology (mitral regurgitation: 70.0% vs 73.8%, mitral stenosis: 7.5% vs 3.8%, and mixed disease: 22.5% vs 22.5%; p=0.6). Most baseline characteristics not included in the matching model were balanced among the TMVI/SMVR cohorts: European System for Cardiac Operative Risk Evaluation (EuroSCORE) II (5.8% [IQR 2.9; 7.5] vs 4.2% [IQR 2.4; 6.8]; p=0.3) and Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score (5.2% [IQR 3.2; 8.6] vs 4.1% [IQR 3.3; 6.1]; p=0.076). Coronary artery disease (67.5% vs 32.5%; p<0.001) and previous percutaneous coronary intervention (47.5% vs 25.0%; p=0.023) differed among groups. Mitral VARC (MVARC) device success at 30 days was achieved in 82.5% of patients after TMVI and 57.5% of patients after SMVR (p=0.04). MVARC procedural success at 30 days was 75.0% after TMVI versus 52.5% after SMVR (p=0.07). Thirty-day mortality (2.5% vs 3.8%; p=0.47), technical success (97.5% vs 97.5%; p=1.0), major bleeding (17.5% vs 18.7%; p=0.087), stroke (5.0% vs 4.9%; p=1.0) and postoperative haemodialysis (7.5% vs 5.2%; p=0.4) were similar in both groups.
CONCLUSIONS: Patients with intermediate surgical risk, according to STS-PROM and EuroSCORE II, demonstrated higher rates of MVARC device at 30 days after TMVI compared to 30 days after SMVR. Rates of survival and procedural success, neurological, renal and bleeding complications were similar. Transfusion count and length of stay were lower after TMVI. For elderly patients at intermediate risk, a TMVI eligibility assessment may be considered.
Transcatheter mitral valve implantation (TMVI) is a less invasive treatment for patients who are ineligible for surgery or at a high surgical risk. While multiple TMVI devices are under investigation in clinical trials, the Tendyne mitral valve system (Abbott) is currently the only commercially available device, having received European conformity (CE) approval in 2020. TMVI with the Tendyne device achieves a sustained elimination of mitral regurgitation at 2 years and a reduction of rehospitalisation for heart failure symptoms1. The role of TMVI compared to edge-to-edge repair and mitral valve surgery is not yet well defined. The SUMMIT trial is currently randomising patients to TMVI with the Tendyne device or mitral valve edge-to-edge repair (ClinicalTrials.gov: NCT03433274). The initial trial design − randomising subjects to TMVI with Tendyne or surgical mitral valve replacement (SMVR) − was modified to the current protocol when edge-to-edge repair received U.S. Food and Drug Administration (FDA) approval. In this study, we aim to compare the early outcomes of TMVI using the Tendyne device with those of SMVR.
Methods
The study was approved by the local ethics committee (2023-359-S-KH) and was conducted in accordance with the Declaration of Helsinki.
PATIENTS AND DATA COLLECTION
Patients who underwent TMVI with the Tendyne mitral valve system were identified from the institutional TMVI database. To obtain a control group with comparable baseline characteristics, all patients who underwent SMVR between 2000 and 2022 were identified from our institutional database. Tricuspid valve repair was the only approved concomitant procedure among the SMVR cohort in the current analysis. Of the 1,278 identified patients, we excluded patients who had received mechanical valves, patients who had undergone emergency mitral valve surgery, patients with infective endocarditis of the mitral valve, and patients with previous mitral valve operations. Subsequently, 504 SMVR patients remained, of whom 454 had a complete dataset eligible for matching. These patients were matched at a rate of 1 TMVI patient to 2 SMVR patients using a combination of exact, coarsened exact and propensity score matching. The detailed matching protocol is described in the statistical analysis section of this article.
Thirty-day outcomes were reported according to Mitral Valve Academic Research Consortium (MVARC) recommendations2. All patients provided informed consent for the procedure.
TMVI WITH THE TENDYNE MITRAL VALVE SYSTEM
TMVI was performed with transoesophageal echocardiography (TOE) guidance via transapical access under general anaesthesia. Cutting of the anterior mitral valve leaflet (MitraCut technique) was executed as previously described if left ventricular outflow tract obstruction (LVOTO) caused by a long and poorly tethered anterior mitral valve leaflet (AML) was anticipated3. After achieving an intra-annular position of the sealing body, the Tendyne valve was fully released from the catheter, and the tether was anchored to the apical pad. For a more detailed description of the Tendyne implantation procedure, please refer to Supplementary Appendix 1.
SURGICAL MITRAL VALVE REPLACEMENT
SMVR was performed after a full sternotomy or a left anterolateral minithoracotomy. Under cardiopulmonary bypass and cardioplegic cardiac arrest, mitral valve replacement with or without tricuspid valve repair was performed with a biological mitral valve prosthesis.
STATISTICAL ANALYSIS
Continuous variables are reported as mean±standard deviation (SD) or median (interquartile range [IQR]) and were compared using the Student’s t-test. Categorical variables are reported as numbers (percentage) and were compared using the chi-square test or Fisher’s exact test. In-hospital events are reported as incidence (percentage) and presented as odds ratios (ORs) with 95% confidence intervals (CIs). Mortality, stroke, and other safety outcomes at 30 days were calculated using logistic regression or g-computation, and results are presented as ORs or risk ratios (RRs) with 95% CIs. A 2-tailed p-value of <0.05 was considered to indicate statistical significance. Statistical analyses were performed using R software (R Foundation for Statistical Computing).
For matching, a limited set of variables was used: the only included variables were those which had a complete data set in the institutional database. The matching procedure was a combination of exact matching, coarsened exact matching and propensity score matching. Sex, atrial fibrillation and previous surgical aortic valve replacement (SAVR) or coronary artery bypass grafting (CABG) were exactly matched. Coarsened exact matching using 3-year intervals was used for patient age. Mitral valve pathology, body mass index (BMI) and concomitant tricuspid regurgitation were matched by propensity score. For the subset of patients with previous SAVR or CABG, the matching protocol was less stringent in propensity score matching for age and mitral valve pathology and less stringent in exact matching for sex due to the small number of suitable patients in the surgical database. For the distribution of variables before and after matching, please refer to Supplementary Table 1. After matching, all pre- and postoperative variables were reviewed, and operative risk scores were recalculated for the matched patients to ensure optimal data quality. Differences in 30-day mortality and MVARC combined endpoints between the groups were calculated using g-computation. The outcome model used for g-computation was a logistic regression model that included all variables used for matching, as well the variables “diabetes mellitus” and “coronary artery disease”, which were unbalanced among the two groups after matching. The model was used to calculate the average treatment effect on the treated (ATT), using cluster-robust standard error. In particular, the R packages “MatchIt” and “marginaleffects” (R Foundation for Statistical Computing) were used for matching and g-computation.
Results
Between June 2020 and May 2023, 40 patients underwent TMVI with the Tendyne device at the German Heart Center Munich. Among 1,278 patients undergoing SMVR between 2000 and 2022 at our department, the 1:2 matching protocol was performed as mentioned above and resulted in 80 SMVR patients serving as a control group (Central illustration).
Of these, 24 patients (30%) had received concomitant tricuspid valve repair.
The baseline variables (Figure 1, Table 1) included in the matching protocol were well balanced among the groups (TMVI vs SMVR): mean age (78 years [IQR 75; 80] vs 78 years [IQR 73; 80]; p=0.797), female sex (60% vs 60%; p=1.0), atrial fibrillation (67.5% vs 63.7%; p=0.839), previous SAVR (12.5% vs 10.0%; p=0.785), previous CABG (20.0% vs 16.2%; p=0.799), BMI (25.54 kg/m² vs 25.24 kg/m²; p=0.723) and mitral valve pathology (mitral regurgitation: 70.0% vs 73.8%, mitral stenosis: 7.5% vs 3.8% and mixed disease: 22.5% vs 22.5%; p=0.648). Most baseline characteristics not included in the matching model were also balanced (Table 1); among these were left ventricular ejection fraction (p=0.07), pulmonary hypertension (p=0.998), previous myocardial infarction (p=1.0), creatinine levels (p=0.259), chronic obstructive pulmonary disease (COPD; p=1.0), European System for Cardiac Operative Risk Evaluation (EuroSCORE) II (p=0.264) and Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM; p=0.076). Differences among the groups (TMVI vs SMVR) were seen for aetiologies of mitral regurgitation (MR; primary MR: 45.0% vs 69.0%, secondary MR: 17.5% vs 2.5%, mixed aetiology: 27.5% vs 22.5%; p=0.01), diabetes mellitus (27.5% vs 11.2%; p=0.046), prevalence of coronary artery disease (67.5% vs 32.5%; p<0.001) and previous percutaneous coronary intervention (47.5% vs 25.0%; p=0.023).
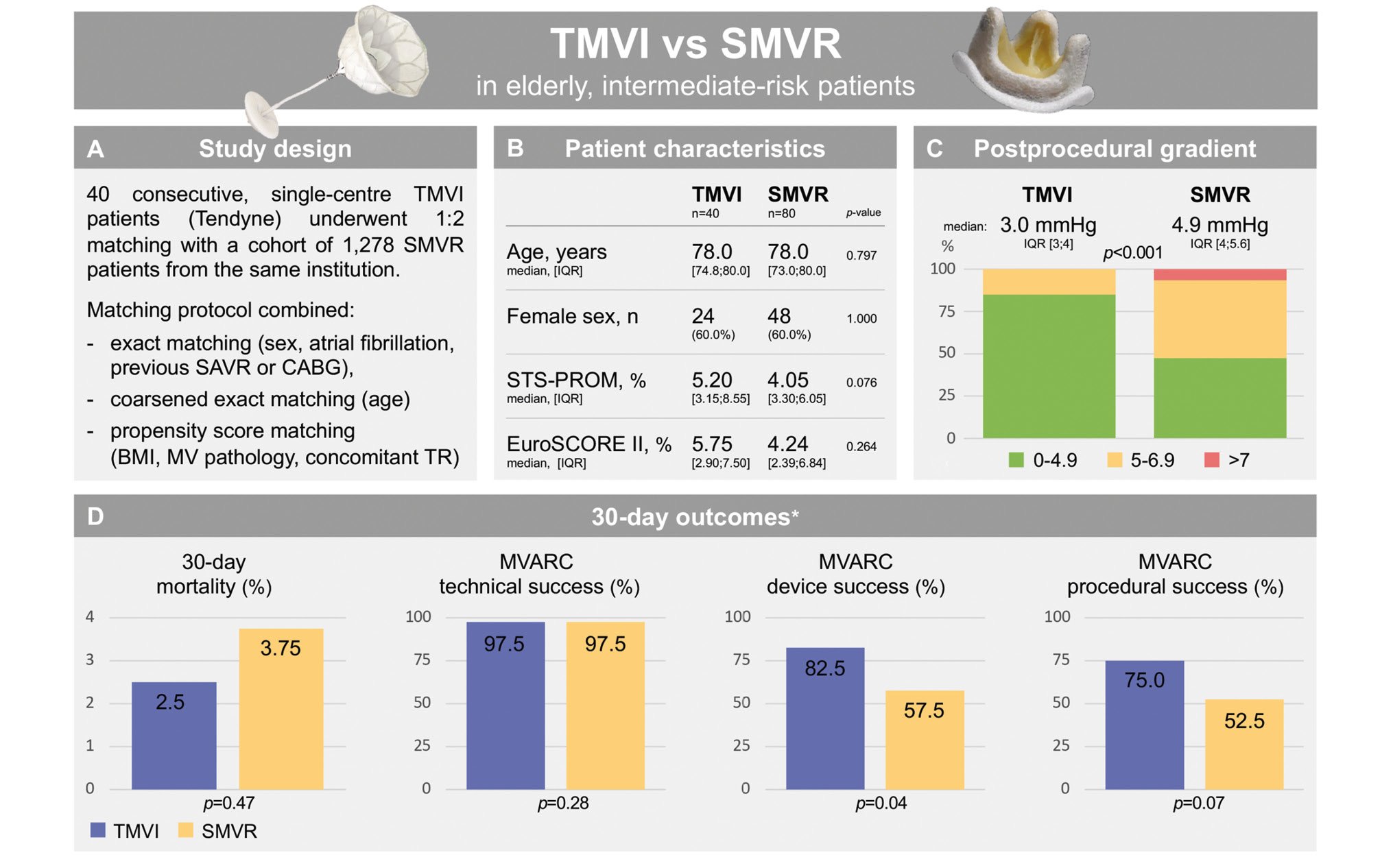
Central illustration. Outcomes of transcatheter mitral valve implantation versus surgical mitral valve replacement in matched elderly patients. *p-values were calculated using g-computation (ATT). A) Study design, B) baseline patient characteristics, C) postprocedural transvalvular gradient and D) 30-day outcomes. ATT: average treatment effect on the treated; BMI: body mass index; CABG: coronary artery bypass grafting; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; MV: mitral valve; MVARC: Mitral Valve Academic Research Consortium; SAVR: surgical aortic valve replacement; SMVR: surgical mitral valve replacement; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TMVI: transcatheter mitral valve implantation; TR: tricuspid regurgitation
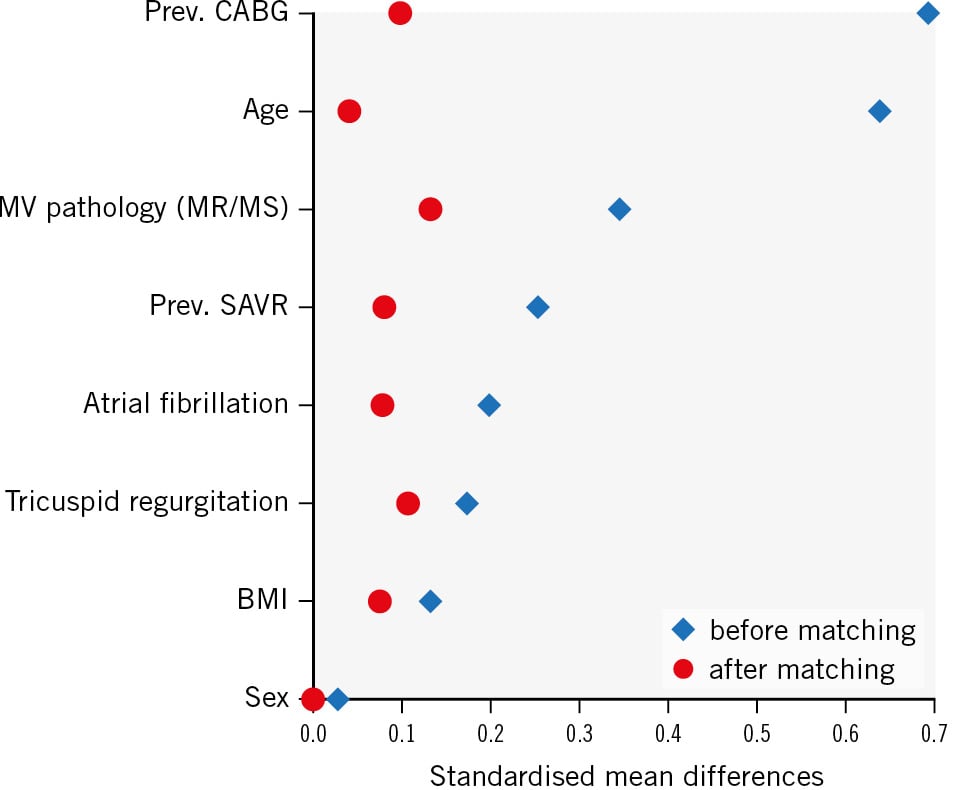
Figure 1. Love plot. Squares and circles depict the standardised mean differences of variables before and after matching. Only variables included in the matching protocol are shown. BMI: body mass index; CABG: coronary artery bypass graft; MR: mitral regurgitation; MS: mitral stenosis; MV: mitral valve; prev.: previous; SAVR: surgical aortic valve replacement
Table 1. Baseline characteristics.
| SMVR n=80 | TMVI n=40 | p-value | |
|---|---|---|---|
| Baseline – patient characteristics | |||
| Female | 48 (60.0) | 24 (60.0) | 1.000 |
| Age, years | 78.0 [73.0; 80.0] | 78.0 [74.8; 80.0] | 0.797 |
| BMI, kg/m2 | 25.2±3.97 | 25.5±4.01 | 0.723 |
| GFR, ml/min | 55.0 [45.5; 75.0] | 50.0 [41.8; 70.5] | 0.469 |
| Creatinine, mg/dl | 1.10 [0.90; 1.30] | 1.20 [0.90; 1.42] | 0.259 |
| Diabetes mellitus | 9 (11.2) | 11 (27.5) | 0.046 |
| Hypertension | 64 (80.0) | 26 (65.0) | 0.118 |
| Coronary artery disease | 26 (32.5) | 27 (67.5) | 0.001 |
| Myocardial infarction | 17 (21.2) | 8 (20.0) | 1.000 |
| Atrial fibrillation | 51 (63.7) | 27 (67.5) | 0.839 |
| Previous stroke | 7 (8.75) | 6 (15.0) | 0.355 |
| COPD | 17 (21.2) | 8 (20.0) | 1.000 |
| STS-PROM, % | 4.05 [3.30; 6.05] | 5.20 [3.15; 8.55] | 0.076 |
| EuroSCORE II, % | 4.24 [2.39; 6.84] | 5.75 [2.90; 7.50] | 0.264 |
| Baseline – echocardiography | |||
| MV disease | 0.649 | ||
| Regurgitation | 59 (73.8) | 28 (70.0) | |
| Stenosis | 3 (3.75) | 3 (7.50) | |
| Mixed | 18 (22.5) | 9 (22.5) | |
| MR grade | 0.435 | ||
| None-mild | 6 (7.5) | 5 (12.5) | |
| Moderate | 6 (7.5) | 2 (5.0) | |
| Severe | 68 (85.0) | 32 (80.0) | |
| MR aetiology | 0.010 | ||
| Primary | 55 (68.8) | 18 (45.0) | |
| Secondary | 2 (2.5) | 7 (17.5) | |
| Mixed | 18 (22.5) | 11 (27.5) | |
| LVEF | 0.071 | ||
| ≥50% | 63 (78.8) | 25 (62.5) | |
| 41-49% | 4 (5.0) | 7 (17.5) | |
| ≤40% | 12 (15.0) | 8 (20.0) | |
| LVEDD, mm | 51.5±10.4 | 53.3±8.94 | 0.505 |
| Systolic PAP, mmHg | 47.0 [40.0; 63.0] | 48.5 [38.2; 66.0] | 0.988 |
| Baseline – previous cardiac procedures | |||
| MV edge-to-edge repair | 3 (3.75) | 0 (0) | 0.550 |
| PCI | 20 (25.0) | 19 (47.5) | 0.023 |
| CABG or SAVR | 20 (25.0) | 10 (25.0) | 1.000 |
| CABG | 13 (16.2) | 8 (20.0) | 0.799 |
| SAVR | 8 (10.0) | 5 (12.5) | 0.758 |
| Data are presented as n (%), median [Q1; Q3] or mean±SD. BMI: body mass index; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GFR: glomerular filtration rate; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MV: mitral valve; PAP: pulmonary artery pressure; PCI: percutaneous coronary intervention; Q1: first quartile; Q3: third quartile; SAVR: surgical aortic valve replacement; SD: standard deviation; SMVR: surgical mitral valve replacement; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TMVI: transcatheter mitral valve implantation | |||
PROCEDURAL DATA
The procedure time for SMVR (median: 217 min [IQR 180; 265], isolated SMVR: 205 min [IQR 176; 252], SMVR and tricuspid repair: 239 min [IQR 209; 286]) was significantly longer than that for TMVI (median: 112 min [IQR 97; 140]; p<0.001). An SMVR with a full sternotomy was performed in 76 patients; a right-sided anterolateral minithoracotomy was performed in 4 patients. Inotropic support was administered in all SMVR procedures and in 27.5% of TMVI procedures (p<0.001). In the SMVR group, 24 patients (30%) received concomitant tricuspid valve repair, while no tricuspid valve procedures were performed in the TMVI group (p<0.001). In 2 TMVI patients, a simultaneous transcatheter aortic valve implantation was performed (1 transapical, 1 transfemoral). A MitraCut of the AML was executed in 2 procedures prior to Tendyne implantation. Apical bleeding required a femoral cardiopulmonary bypass and unloading of the left ventricle to achieve haemostasis in 1 procedure. Throughout all the TMVI procedures, there was no embolisation of the Tendyne valves, no retrieval of the prosthetic valve was required and no intraprocedural LVOTO occurred; the mean left ventricular outflow tract (LVOT) gradient measured 6.7±6.3 mmHg. MVARC technical success was similar after TMVI (97.5%) and after SMVR (97.5%; p=0.28) (Table 2).
Table 2. Thirty-day mortality and MVARC composite endpoints.
| SMVR n=80 | TMVI n=40 | Risk ratio (95% CI) | p-value | |
|---|---|---|---|---|
| 30-day mortality | 3(3.75) | 1(2.50) | 0.66 (0.21-2.07) | 0.47 |
| Technical success | 78(97.5) | 39(97.5) | 0.98 (0.93-1.02) | 0.28 |
| Device success | 46(57.5) | 33(82.5) | 1.38 (1.01-1.89) | 0.04 |
| Procedural success | 42(52.5) | 30(75.0) | 1.37 (0.96-1.95) | 0.07 |
| Data are presented as n (%). Risk ratios were calculated using g-computation. CI: confidence interval; MVARC: Mitral Valve Academic Research Consortium; SMVR: surgical mitral valve replacement; TMVI: transcatheter mitral valve implantation | ||||
IN-HOSPITAL AND 30-DAY OUTCOMES
Table 2 shows 30-day mortality rates and MVARC composite endpoints. MVARC device success at 30 days was achieved in 82.5% of patients after TMVI and in 57.5% of patients after SMVR (p=0.04).
MVARC procedural success at 30 days was achieved in 75% of patients after TMVI and in 52.5% of patients after SMVR (p=0.07) (Central illustration). Table 3 displays the clinical outcome at 30 days after TMVI and SMVR and the single endpoints defining MVARC device and procedural success. Patients had shorter lengths of stay in the intensive care unit (p<0.001) and in hospital (p=0.004) after TMVI compared to SMVR. The red blood cell transfusion count was lower after TMVI compared to after SMVR (p<0.001). The transprosthetic mitral gradient (Figure 2) measured 3 mmHg (IQR 3; 4) after TMVI and 4.9 mmHg (IQR 4.0; 5.6) after SMVR (p<0.001). Trace (TMVI 5%, SMVR 0%) and mild paravalvular leakage (TMVI 5%, SMVR 0%) differed among the groups (p=0.005). The mean LVOT gradient after TMVI measured 4.5 mmHg [IQR 3.0; 9.3]. Thirty-day mortality, major bleeding complications, stroke, and renal failure requiring haemodialysis were comparable among groups.
One patient developed a significant LVOTO after TMVI which was related to the systolic anterior motion of the anterior mitral valve leaflet and required LVOT stent-graft implantation on day 21 after the procedure.
Table 3. Clinical outcome at 30 days.
| SMVR n=80 | TMVI n=40 | p-value | |
|---|---|---|---|
| Length of stay | |||
| ICU LOS, days | 5.0 [2.5; 7.0] | 1.0 [1.0; 4.75] | 0.001 |
| Hospital LOS, days | 12.5 [9.75; 16.0] | 9.0 [7.0; 13.0] | 0.004 |
| Adverse events | |||
| 30-day stroke | 3 (4.9) | 2 (5.0) | 1.000 |
| Bleeding | 0.087 | ||
| BARC Type 3a | 2 (2.7) | 5 (12.5) | |
| BARC Type 3b | 6 (8.0) | 1 (2.5) | |
| BARC Type 4 | 6 (8.0) | 1 (2.5) | |
| RBC transfusion, units | 2.0 [2.0; 4.0] | 1.0 [0.0; 2.0] | <0.001 |
| Major cardiac structural complications* | 4 (5.0) | 1 (2.5) | 0.663 |
| Renal failure requiring HD | 12 (15.2) | 3 (7.5) | 0.367 |
| Severe heart failure† | 4 (5.0) | 2 (5.0) | 1.000 |
| Prosthetic valve malposition | 0 (0) | 0 (0) | 1.000 |
| Unplanned surgical intervention‡ | 8 (10.0) | 0 (0) | 0.051 |
| Myocardial infarction | 0 (0) | 0 (0) | 0.000 |
| MR >1 | 1 (1.25) | 1 (2.5) | 1.000 |
| Transmitral gradient >5 mmHg | 23 (28.75) | 5 (12.5) | 0.066 |
| Data are presented as n (%) or median [IQR]. *Major cardiac structural complication including cardiac perforation resulting in death, life-threatening bleeding, haemodynamic compromise or tamponade, or requiring unplanned surgical or percutaneous intervention. †Severe postoperative heart failure, hypotension, or respiratory failure. ‡Unplanned surgical or interventional procedure related to the device or access procedure. BARC: Bleeding Academic Research Consortium; HD: haemodialysis; ICU: intensive care unit; IQR: interquartile range; LOS: length of stay; MR: mitral regurgitation; RBC: red blood cells; SMVR: surgical mitral valve replacement; TMVI: transcatheter mitral valve implantation | |||
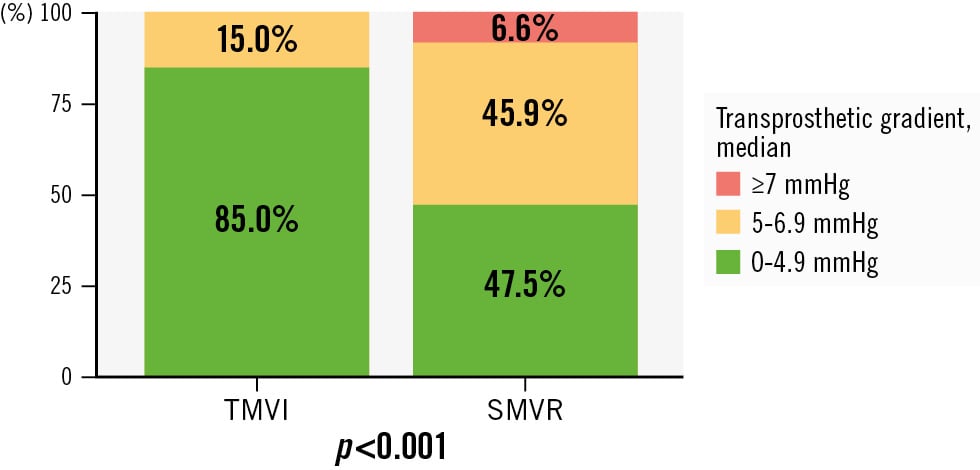
Figure 2. Transprosthetic gradient after TMVI and SMVR. SMVR: surgical mitral valve replacement; TMVI: transcatheter mitral valve implantation
Discussion
This is the first study comparing TMVI with the Tendyne mitral valve system to SMVR. The main findings of our study are as follows
- TMVI achieved higher MVARC device success at 30 days compared to SMVR.
- Thirty-day mortality was comparable after TMVI (2.5%) and SMVR (3.8%).
- Bleeding complications (Bleeding Academic Research Consortium >Type 3a), stroke and renal failure were similar after TMVI and SMVR.
- Intensive care unit length of stay and hospital length of stay were shorter after TMVI than SMVR.
SURVIVAL
Thirty-day mortality after SMVR has been reported at 4.0% in younger patients (mean age 69 years) suffering from secondary mitral regurgitation (SMR) and between 9.2% and 19.0% in elderly patients (mean age 78-83 years) with predominantly primary mitral valve disease45678. Comparison of previously reported mortality data with the 3.8% short-term mortality of our SMVR cohort is biased by the younger age of the SMR patients and by the rates of concomitant aortic valve or CABG procedures, which ranged from 34% to 48% and 55% to 75%, respectively, in previous studies45678.
Mortality at 30 days after TMVI with the Tendyne device was 2.5% in our cohort, whereas previous studies reported higher 30-day mortality ranging from 6% to 10.2% and 12%91011. These patients were younger (mean age 75 years91011), showed a higher prevalence of SMR (89%, 69% and 37%) and had a higher STS-PROM score (6.5%, 7.7% and 7.8%) while still resulting in an intermediate operative risk profile91011.
IN-HOSPITAL ADVERSE EVENTS AFTER TMVI AND SMVR
The stroke rate (5%, n=2) of our TMVI cohort at 30 days was higher than the 2-3% reported in previous TMVI studies91011. It is worthy of note that 1 patient from our cohort had suffered at least 2 strokes prior to TMVI, significantly increasing his perioperative stroke risk12. The occurrence of perioperative stroke after TMVI was similar to that in the SMVR control group (4.9%) and comparable to previously reported SMVR data ranging from 3.1% to 5.0%456.
Renal failure requiring haemodialysis has been reported to be considerably higher (13.0% to 19.6% of patients) after SMVR compared to after TMVI (5% to 8% of patients)67911. Our data (7.5% of TMVI patients vs 15.2% of SMVR patients) are in line with these findings. However, the difference in renal failure between TMVI and SMVR patients did not reach statistical significance.
In our study, the occurrence of MVARC major bleeding complications was comparable between the TMVI (17.5%) and SMVR (18.7%) cohorts. For TMVI, 2 previous studies reported a rate of 20% for major bleeding events, similar to our results910. The TENDER registry reported lower rates of major bleeding complications (11%)11. Previous SMVR studies have not reported bleeding complications following standardised definitions. They indicate either total volume of red blood cell transfusion, the rethoracotomy rate or the bleeding complication rate678.
We found that hospital length of stay was significantly shorter after TMVI than after SMVR (9.0 days vs 12.5 days). Compared to our data, previous studies reported longer stays after TMVI (11 days) and after SMVR (13 days to 19 days), indicating an overall pattern of faster recovery after TMVI compared to SMVR569. Potential economic advantages, and higher patient acceptance and satisfaction need further analyses.
At the end of the procedure, MVARC technical success was 97.5% for both TMVI and SMVR. Previous studies with the Tendyne device report >95% technical success rates, in line with our findings91011. The MVARC composite endpoints, device success and procedural success, were achieved more often at 30 days after TMVI compared to 30 days after SMVR. Procedural success at 30 days was 75% in our TMVI cohort, which is comparable to the 80% procedural success rate reported from the TENDER registry11. To date, only sparse data exist regarding the outcomes with dedicated TMVI devices. In the early feasibility study, successful implantation of the 35 Fr transseptal Intrepid device (Medtronic) was reported in 14 of 15 patients13. Technical success among 30 patients treated with the transseptal HighLife device (HighLife Medical) was 90%14. The EVOQUE TMVR system (Edwards Lifesciences) achieved 93% technical success in the first 14 patients treated with the device15. The CHOICE-MI registry included 229 patients treated with 10 dedicated TMVI devices and reported a rate of 95.2% for technical success16.
CURRENT TMVI CHALLENGES AND FUTURE PERSPECTIVES
In the treatment of mitral valve disease, the role of TMVI alongside other treatment modalities needs to be defined more clearly. TMVI is currently limited to patients who are unsuitable for surgery and who present with a mitral valve morphology that is ineligible for edge-to-edge repair. Currently, a significant number of patients are ineligible for TMVI as mitral valve devices either would not cover the mitral annulus dimensions or would reduce the LVOT area to below the threshold that can cause LVOTO. This study is the first to compare TMVI with SMVR. Clearly, prospective randomised studies comparing TMVI and SMVR are needed to confirm these initial findings. The SUMMIT trial, which is currently enrolling patients, aims to compare TMVI using the Tendyne system with edge-to-edge repair of the mitral valve.
Several transcatheter mitral valve systems with a transseptal delivery route are currently under clinical investigation and might broaden the clinical use of TMVI after achieving the relevant authorities' approval. Additional long-term TMVI data on valve durability are required for future discussion on TMVI as a potential substitute for SMVR in elderly patients.
Limitations
Several limitations need to be addressed regarding this study. First, the retrospective nature of this study has inherent biases. The 20-year period of SMVR patient inclusion optimised the patient selection to the matching protocol, but patient outcome might be biased because of modifications or improvements in postoperative care. There was no prespecified protocol, and only observational data could be included in the analysis. Data completion and revision, including operative risk score calculation, were partially conducted after matching, for the matched collective only. Thus, matching was performed on a parsimonious dataset. This resulted in some imbalances of variables not included in matching, namely MR aetiology, diabetes and coronary artery disease. According to these variables, patients receiving TMVI were slightly more unwell. However, considering other variables which were added after matching, such as EuroSCORE II or the glomerular filtration rate, the populations appear to be reasonably matched. The echocardiographic results were not core laboratory adjudicated, and serial echocardiography studies at discharge and at 30 days were only available for a limited number of patients. Follow-up is currently limited to 30 days to ensure the completeness of follow-up.
A rigid TMVI screening protocol which is predominantly driven by four-dimensional computed tomography (CT) and focuses on intracardiac anatomy and morphology results in a highly selected TMVI patient cohort. As the decision to opt for SMVR is not based on CT imaging, potential differences in intracardiac anatomy and morphology (i.e., mitral valve annulus size, mitral annulus calcification) among groups are not subject to retrospective analysis. MVARC criteria were developed to standardise outcome reporting after interventional mitral valve procedures, and their applicability to SMVR needs future evaluation.
Conclusions
Patients with intermediate surgical risk, according to STS-PROM and EuroSCORE II, demonstrated higher rates of MVARC device at 30 days after TMVI compared to 30 days after SMVR. Rates of survival and procedural success, neurological, renal and bleeding complications were similar. Transfusion count and length of stay were lower after TMVI. For elderly patients at intermediate risk, a TMVI eligibility assessment may be considered.
Impact on daily practice
In this retrospective study, transcatheter mitral valve implantation achieved higher Mitral Valve Academic Research Consortium device and procedural success compared to surgical mitral valve replacement (SMVR). These are promising data for an evolving technology which is being developed as a complementary therapy to mitral edge-to-edge repair and SMVR. Future randomised trials are required to confirm our findings.
Conflict of interest statement
H. Ruge serves as a physician proctor for and is an advisory board member of Abbott. M. Krane is a physician proctor and a member of the medical advisory board for JOMDD; a physician proctor for Peter Duschek; a medical consultant for Evotec and Moderna; and has received speaker honoraria from Medtronic and Terumo. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

