Introduction
Transcatheter mitral valve implantation (TMVI) has recently emerged as a treatment option for selected high-risk patients with symptomatic mitral regurgitation (MR).
Although the feasibility of TMVI in patients with previous aortic valve (AV) prostheses has been reported1, the interaction between a TMVI and an AV prosthesis has not previously been well characterised. In particular, the risk of left ventricular outflow tract (LVOT) obstruction after TMVI may be higher in patients with a pre-existing aortic valve prosthesis, due to the presence of concomitant LV hypertrophy. Moreover, the frame of the aortic prosthesis can extend into the LVOT, and the anchoring mechanism of TMVI may interfere with the normal functioning of an aortic prosthesis. Consequently, the presence of an aortic prosthesis has been considered a relative contraindication to TMVI in these patients and represented an exclusion criterion in most of the TMVI early feasibility trials.
The Tendyne™ mitral valve system (Abbott Vascular, Santa Clara, CA, USA) is a fully retrievable and repositionable TMVI system, which consists of a circular inner frame with porcine pericardial leaflets mounted on a self-expanding outer frame and is anchored via a tether to an epicardial pad through apical access2,3.
Methods
PATIENTS
This is a retrospective analysis of the baseline, periprocedural, and post-procedural data collected on 11 consecutive patients with previous surgical aortic valve replacement (AVR) or transcatheter aortic valve implantation (TAVI) who underwent subsequent TMVI using the Tendyne system.
Eligibility for Tendyne implantation was determined by the site Heart Team in consultation with the Tendyne clinical team, including the core laboratory analyses of the echocardiographic studies (transthoracic echocardiography [TTE] and transoesophageal echocardiography [TEE]), and computed tomography (CT).
POSTOPERATIVE EVALUATION
Echocardiographic evaluation included assessment of performance of aortic and mitral valve prostheses and left and right ventricular function. Mitral valve performance was characterised by central and paravalvular MR, stability and integrity of the device, haemolysis, thrombosis or endocarditis, mitral stenosis (mitral valve [MV] gradient ≥6 mmHg and effective orifice area ≤1.5 cm2), haemodynamically relevant LVOT obstruction (defined as ≥10 mmHg increase in LVOT gradient vs baseline), damage to surrounding cardiac structures, or need for permanent pacemaker implantation. Safety endpoints included mortality, stroke, myocardial infarction, life-threatening bleeding, major vascular complications, renal failure requiring dialysis, and other device- or procedure-related serious adverse events.
Results
BASELINE CHARACTERISTICS
The study population included 11 patients with prior surgical AVR (5/11 patients, 45.5%) or TAVI (6/11 patients, 54.5%) who underwent subsequent TMVI with the Tendyne valve. The baseline and demographic data of these patients are summarised in Supplementary Table 1.
PERIPROCEDURAL OUTCOMES
Procedural outcomes are listed in Supplementary Table 2. The Tendyne prosthesis was successfully implanted in all patients with no intraprocedural complications. No dysfunction of the aortic prosthesis was observed following the Tendyne implant. Technical success was achieved in all patients (11/11 patients, 100%), with MR reduction to grade ≤1+ in 100% of the cases. No cases of clinically significant LVOT obstruction occurred.
FOLLOW-UP DATA
One patient died within 30 days due to persisting severe right heart failure after the procedure. During follow-up (mean 305 days), all-cause mortality was 27% (3/11 patients: 2 cardiovascular deaths, and 1 non-cardiovascular death). There were no reported strokes, myocardial infarctions, heart failure hospitalisations, or reinterventions related to the MV. No valve-related adverse events were observed. At last echocardiography, in all cases the Tendyne prosthesis was functioning normally, with no cases of late valve embolisation or malposition, or evidence of mitral stenosis (mean transmitral gradient 4±2 mmHg). There were no patients with new intravalvular or paravalvular regurgitation in the aortic position, and no significant changes in transvalvular gradient, demonstrating preserved function of the aortic prosthesis. Also, no cases of hypoattenuated leaflet thickening (HALT) or stent deformation of the aortic prostheses were observed at follow-up.
Discussion
This is the first report of outcomes of TMVI with the Tendyne system in patients with previous surgical AVR or TAVI. These results are similar to the previously published outcomes of Tendyne in the first 100 patients, which excluded patients with an existing aortic prosthesis, and where reported procedural success was 96%. Despite the limited sample size, the comparable results reported in the present series suggest that the presence of a previously implanted aortic prosthesis does not represent a technical issue precluding implantation of the Tendyne valve, and therefore should not represent an exclusion criterion during the patient selection process (Figure 1). Feasibility has been shown in both biological and mechanical surgical prostheses, as well as with transcatheter ones (including both balloon-expandable and self-expanding) (Supplementary Table 1). In particular, no cases of interaction between the mitral and the aortic prosthesis have been observed. Based on the effective MR reduction and absence of valve-related adverse events, the safety profile of the Tendyne system in the presence of an aortic prosthesis is supported in this limited series. This lack of interaction with the aortic prosthesis is not surprising based on the sealing and fixation method of the Tendyne valve, which differs from other devices. Tendyne uses an apical pad for fixation, rather than relying on interaction with the mitral annulus. In summary, our series demonstrated that, with careful preprocedural planning, patients with a previous aortic prosthesis could also be eligible for Tendyne, without any specific concerns.
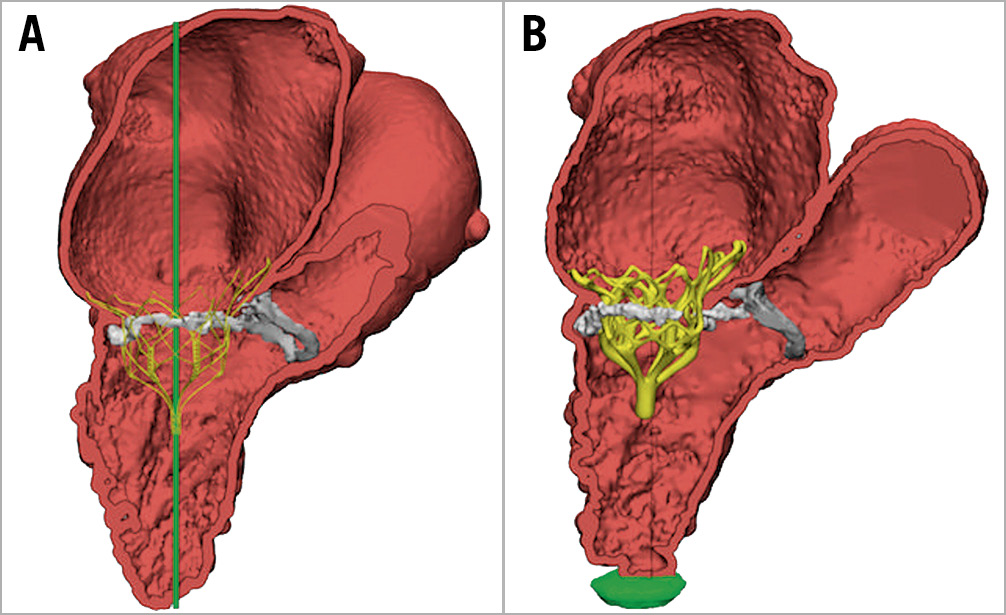
Figure 1. CT analysis of the predicted (A) valve implantation compared to the actual (B) result in a patient with a Trifecta bioprosthetic surgical valve and severe mitral annular calcification (MAC).
The retrievability and repositionability features of the Tendyne valve represent a further advantage compared to other platforms, especially in the specific setting of challenging anatomies, allowing the operator to remove the valve in case of LVOT obstruction, aortic prosthesis dysfunction, or suboptimal outcomes (Figure 2).
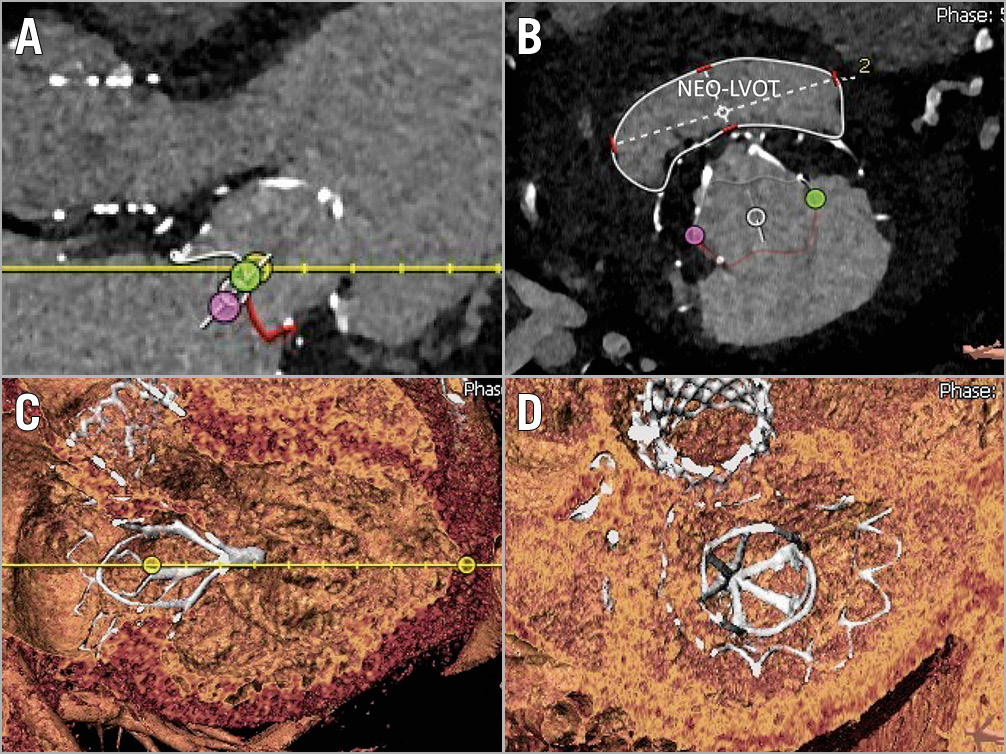
Figure 2. CT-scan reconstruction. A) The open LVOT after the implantation of the Tendyne prosthesis. Neo-LVOT area at the narrowest level was 984.1 mm2 (B). C) & D) 3D reconstructed long-axis and short-axis views, respectively, from the postoperative CT scan of the same patient.
Limitations
Absence of a central core lab to adjudicate the outcomes, the small size of the sample and the absence of a control group represent the major limitations of this study.
Conclusion
TMVI with the Tendyne system in patients with a pre-existing aortic valve prosthesis is feasible and safe, and provides effective MR elimination. The presence of an aortic prosthesis, either surgical or transcatheter, did not result in procedural complications or alter the function of either prosthesis and therefore this should not be considered as a contraindication to the procedure.
|
Impact on daily practice The results of this small series show the feasibility of transapical TMVI with the Tendyne in patients who previously underwent TAVI or surgical AVR, which was considered an exclusion criterion from the feasibility study. |
Appendix. Study collaborators
Benjamin Sun, MD; Minneapolis Heart Institute Foundation at Abbott Northwestern Hospital, Minneapolis, MN, USA. Francesco Maisano, MD; University Hospital of Zurich, University of Zurich, Zurich, Switzerland.
Conflict of interest statement
M. Taramasso is a consultant for Abbott, Boston Scientific, 4Tech, and CoreMedic, and has received speaker fees from Edwards Lifesciences. P. Sorajja is a consultant or advisory board member for Boston Scientific, Edwards Lifesciences, Admedus, Medtronic, Gore, and Abbott Structural Heart. G. Dahle is a consultant for Abbott. N. Dumonteil has received consulting fees/honoraria from Boston Scientific, Edwards Lifesciences, Medtronic, and Biotronik. U. Schäfer is a consultant for Abbott Vascular. T. Modine is a consultant for Abbott Vascular. G. Nickenig declares honoraria for lectures/advisory boards/clinical trials/research funding from Abbott, AGA, AstraZeneca, Bayer, Berlin Cardiovalve, Chemie, Biosensus, Biotronik, BMS, Boehringer Ingelheim, Daiichi Sankyo, Edwards, Medtronic, Novartis, Pfizer, Sanofi-Aventis, and St. Jude. V. Babaliaros is a consultant for Edwards Lifesciences. L. Conradi is a consultant for Abbott and Edwards Lifesciences. B. Sun is a consultant for Abbott. F. Maisano declares grant and/or research support from Abbott Vascular, Medtronic, Edwards Lifesciences, Biotronik, and Boston Scientific Corporation, declares consulting fees or honoraria from Abbott Vascular, Medtronic, Edwards Lifesciences, Perifect, Xeltis, Transseptal Solutions, and Cardiovalve, and has royalty income/IP rights from Edwards Lifesciences and 4Tech. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

