Device description
Name and manufacturer: Tendyne System; Tendyne, Roseville, MN, USA (Figure 1).

Figure 1. Tendyne transcatheter mitral valve.
Approval status: Clinical approval has not yet been granted; a global early feasibility trial is currently enrolling.
Platform: Symmetrical trileaflet valve comprised of gluteraldehyde-treated porcine pericardium, two nitinol stents, adjustable tether and apical fixation/sealing pad.
Delivery components: Guide catheter, delivery/retrieval catheter, slide-locking system, left ventricular apical tethering device and gauge.
Specific design: Valve sewn within two self-expanding nitinol stents. The inner stent is one size and circular to maintain consistent, larger effective orifice area >3.0 cm2; the outer stent is D-shaped to conform to the shape of the native mitral annulus. Atrial flange offers atrial sealing and anchoring. Left ventricular apical tethering system with apical pad reduces paravalvular insufficiency and assists with apical closure.
Delivery approach/system: Transapical 34 Fr (outer diameter) sheath.
Device sizes: Multiple configurations to treat varying mitral valve anatomies, ranging from a septal-lateral (anterior to posterior annulus or AP) diameter of 30-43 mm and an intercommissural diameter (commissure to commissure) of 34-50 mm.
Procedural details
The Tendyne device is designed to treat degenerative (primary) or functional (secondary) mitral regurgitation (MR). Rapid ventricular pacing and cardiopulmonary bypass are not required for Tendyne implantation1. Valve implantation is TEE-guided.
TRANSAPICAL APPROACH
The room and patient are prepared for a transapical thoracotomy. The left ventricular apex is accessed using a 34 Fr delivery sheath, which enters at a 90 degree angle to the mitral annular plane (Online Figure 1A).
DEVICE ENTRY INTO THE LEFT ATRIUM
A balloon-tipped catheter is floated over a guidewire and advanced into the left atrium. The sheath is then advanced to the mid-orifice of the mitral valve. This critical step ensures that the device is free of the subvalvular apparatus, preventing distortion of the papillary muscles and chordae tendinae (Online Figure 1B, Online Figure 1C). The delivery system is advanced in the sheath, which employs a slide-locking system to minimise blood loss.
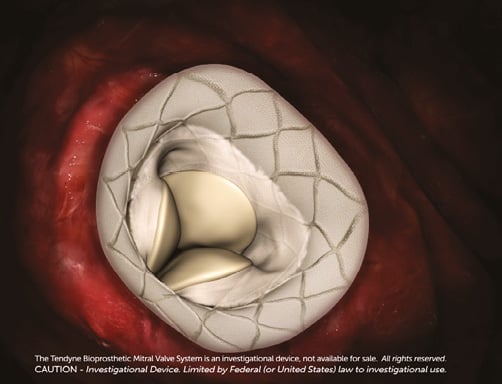
The valve is brought into the left atrium and positioned above the mitral annulus (Figure 2). Moat and colleagues reported that the valve is ideally advanced and allowed to expand until approximately 80% of it is in the left atrium as it may be difficult to pull back the fully expanded Tendyne valve into the minimally compliant mitral valve annulus1,2.
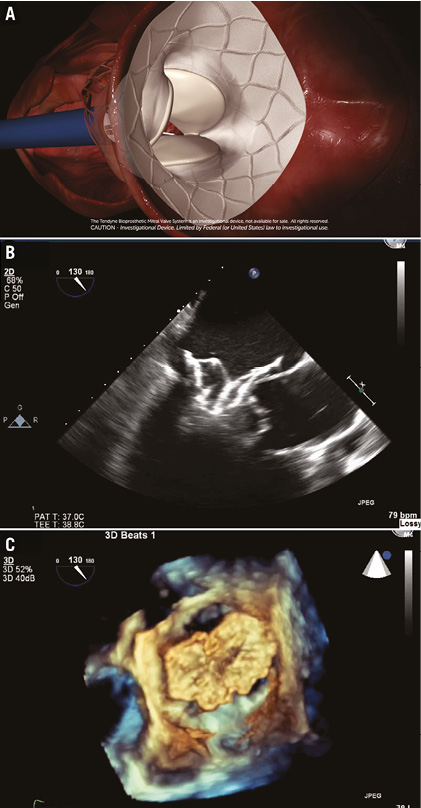
Figure 2. Expansion of the Tendyne valve in the left atrium. A) Expansion of the Tendyne valve in the left atrium. The valve is advanced to approximately 80% expansion. B) & C) 2D and 3D TEE confirmation of the Tendyne valve expansion in the left atrium.
INTRA-ANNULAR VALVE DEPLOYMENT
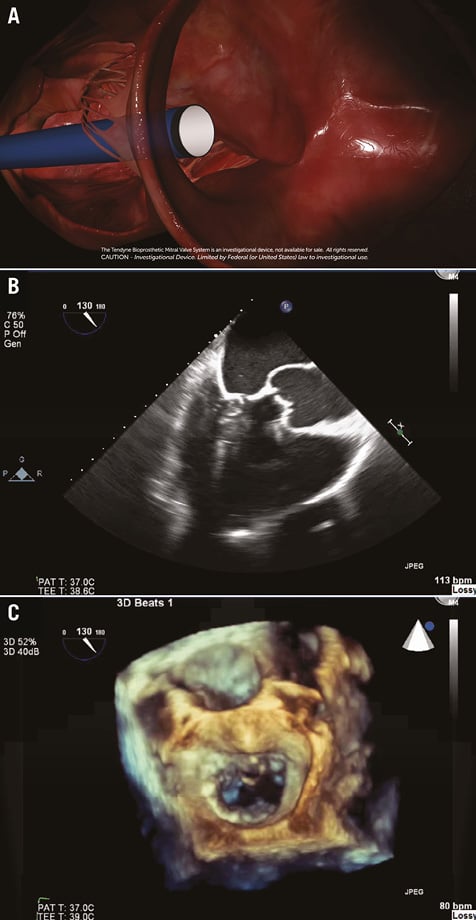
The D-shaped Tendyne outer stent should be oriented such that the straight side and atrial cuff are aligned with the aortomitral continuity and aorta. To obtain the correct orientation, the device is rotated in the left atrium before the atrial cuff has fully expanded1,2. The valve is retracted into the mitral annulus (Online Figure 2), a manoeuvre that produces tactile feedback and should be confirmed by TEE. Proper valve seating should be confirmed based on orientation and intra-annular deployment; the valve should not lift or rock and should be well seated on the atrial floor1,2.
APICAL PAD FIXATION AND ADJUSTMENT OF TETHER TENSION
The apical pad is inserted in position over the tether. A tension gauge tool is used to adjust the tether tension from the valve to the left ventricular apex. Tether tension adjustment is guided by TEE assessment of valve function, device orientation, presence or absence of paravalvular insufficiency (Online Figure 3), aortic root geometry and aortic valve function, and left ventricular posterior wall motion and function2. The valve can be fully repositioned and retrieved even after full deployment. The completely expanded valve is well seen on fluoroscopy (Online Figure 4). The apical pad is secured and assists with closure of the apex (Online Figure 5).
Clinical data
Upon U.S. Food and Drug Administration (FDA) issuance of an 801(e) export permit for unapproved medical devices, the first Tendyne implants took place in Paraguay with oversight from the International Organisation for Standardisation. Two patients undergoing surgical MVR first received a Tendyne implant and were monitored for two hours post implant before the Tendyne valve was removed and surgical MVR commenced. The first patient was a 57-year-old male with New York Heart Association (NYHA) Class IV CHF and severe primary MR due to P2 prolapse; the second patient was a 55-year-old woman with NYHA Class III CHF and severe primary MR due to rheumatic and myxomatous valve disease. Improvement in MR severity and reduction in left atrial pressures and pulmonary artery systolic pressures were observed. Coronary artery restriction, LVOT obstruction, and systolic anterior motion (SAM) of the mitral valve were not seen in either patient3.
Following this, three patients in the United Kingdom were treated under a compassionate use study protocol4. Each patient had significant comorbidities and severe MR with NYHA Class III-IV CHF necessitating hospital admission. All three patients survived the procedure and were discharged home after a mean hospital length of stay of approximately 10 days. All were alive and reported significant symptomatic relief at 30 days4.
Ongoing studies
The Tendyne global early feasibility trial is currently enrolling in Australia (St. Vincent Hospital, Sydney, Australia) and the USA (Abbott Northwestern, Minneapolis, MN, USA): it has enrolled seven patients to date. Thus, 10 patients with symptomatic, severe MR (degenerative MR=3; functional MR=7) have undergone TMVR with the Tendyne valve. Successful implants were performed in 9 out of 10 of patients; 100% were free of intraprocedural death, stroke, or device displacement. All 10 patients were alive at 30 days, 90% of these patients being discharged home2.
In August 2015, Abbott Pharmaceuticals announced an agreement to acquire Tendyne, which is expected to close in the third quarter of 2015. Abbott Vascular also owns MitraClip® (Evalve, Menlo Park, CA, USA), the sole device with FDA approval and globally the most widely used device for transcatheter mitral valve repair.
Unique features
– The only transcatheter mitral valve with a left ventricular apical tethering system and apical pad, designed to provide valve stability, reduce paravalvular insufficiency, and assist with apical closure.
– Inner circular stent with larger orifice area than any surgical valve (>3.0 cm2) and outer D-shaped stent with multiple sizes to fit the native mitral valve annulus.
Potential improvements
– Reduction of overall valve profile to increase LVOT clearance and decrease risk of systolic anterior motion of the mitral valve.
– Reduction of delivery catheter size, currently designed to accommodate the largest valve. Commercial offering will include three delivery catheter sizes, corresponding to valve size.
– Simplification of delivery system (to be used in CE trial) to improve control and predictability.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online Figure 1. Access of the left ventricular apex by the Tendyne system. A) Tendyne delivery sheath accessing the left ventricular apex, perpendicular to the mitral valve annular plane. B) 2D long-axis view TEE confirmation of the delivery system approaching the mitral valve, free of the subvalvular apparatus. C) 3D en face TEE confirmation of the delivery system entering the mitral valve, mid-orifice.
Online Figure 2. Orientation and seating of the Tendyne valve in the mitral annulus. The straight side of the D-shaped outer stent should align with the aorta and anterior mitral annulus, conforming to the natural shape of the mitral valve. Upon acquisition of the correct orientation, the valve is pulled back into the annulus for deployment.
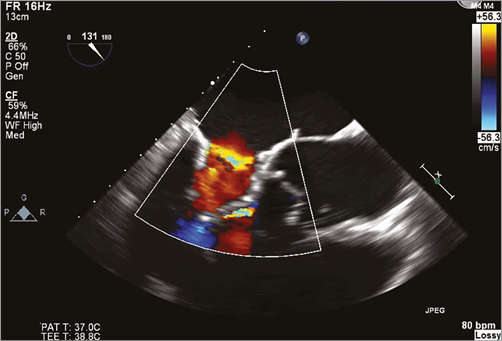
Online Figure 3. Assessment of paravalvular insufficiency and adjustment of apical tethering by 2D TEE long-axis view.
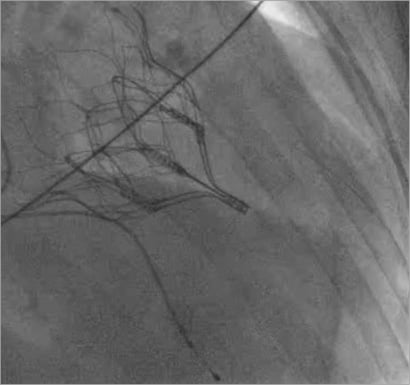
Online Figure 4. Fully expanded and deployed Tendyne valve on fluoroscopy.
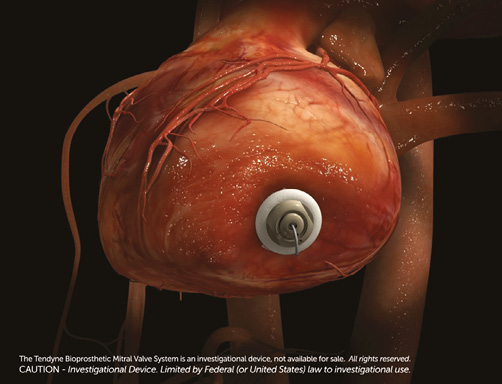
Online Figure 5. Left ventricular apical pad and closure.

