Tendyne device design
The Tendyne valve (Abbott Vascular, Santa Clara, CA, USA) (Figure 1) is designed to be implanted into a beating heart through a left minithoracotomy using a transapical approach. The Tendyne valve has three porcine pericardial tissue leaflets sewn onto an inner circular stent. The inner stent is made from nitinol, a nickel-titanium alloy that has self-expanding properties and is radiopaque. The inner stent is joined to an outer nitinol stent that is covered in porcine pericardium with a polyethylene terephthalate (PET) fabric cuff that provides the sealing surface within the native annulus. The outer stent is formed into a D-shaped body, which is designed to conform to the patient’s native mitral orifice. The cuff is raised along the straight leg of the “D” shape with the intention of orientating this aspect of the cuff against the aortic-mitral continuity. The porcine pericardial tissue is cross-linked and decellularised in a buffered glutaraldehyde solution before being sutured to the inner and outer stents.

Figure 1. Tendyne device.
Together, the inner and outer stent form a self-expanding prosthesis which is connected to a braided fibre tether made of ultra-high molecular weight polyethylene. The tether is designed to stabilise the valve by passing through the left ventricular apex where it is secured to a pad that rests on the epicardial surface. This pad is a polyether ether ketone button covered in PET fabric, designed to promote ingrowth. During implantation, the tether is used to adjust tension and optimise the position of the valve within the mitral annulus. There is an “apical pad tool” that is designed to fasten the apical pad to the braided tether. This tool has an articulating head to facilitate proper angulation, and a cam rotator that drives a pin through the braided tether. The cam rotator allows unlocking and relocking of the pin in case the tension on the tether needs to be adjusted.
Thus, the Tendyne valve is designed to rest on the floor of the left atrium, providing countertraction to the tether force produced by the tether to resist migration of the valve into the left atrium. The device is fully repositionable and retrievable.
Tendyne procedure
The Tendyne valve size is chosen based on mitral annular dimensions derived from multislice computed tomography (MSCT) imaging (Figure 2A) and confirmed by intraoperative three-dimensional transoesophageal echocardiography (3D TOE) (Figure 2B). The device is implanted under general anaesthesia via a transapical approach through a small left minithoracotomy. All the manoeuvres are effected using 2D and 3D TOE imaging guidance. The optimal apical puncture site is determined such that it is perpendicular to the mitral annulus. A soft 0.035” wire is inserted into the left atrium and a balloon-tipped catheter advanced into the left atrium to ensure that the wire is not entrapped in the mitral subvalvar apparatus (Moving image 1). A 34 Fr sheath is advanced over the soft wire into the left atrium. The implant device is advanced into the sheath and then extruded until the outer nitinol frame expands to about 85% of its final deployed size (Moving image 2). The correct anatomical position of the D-shaped outer stent and the raised section of the atrial cuff are then determined and optimised if necessary by rotating the device. The device is then withdrawn towards the ventricle, into an intra-annular position, and deployment is completed (Moving image 3). The apical pad is inserted into position over the tether. The tension on the tether is then adjusted to optimise the position of the device. Finally, the pin is driven through the braided tether to fix it in position.
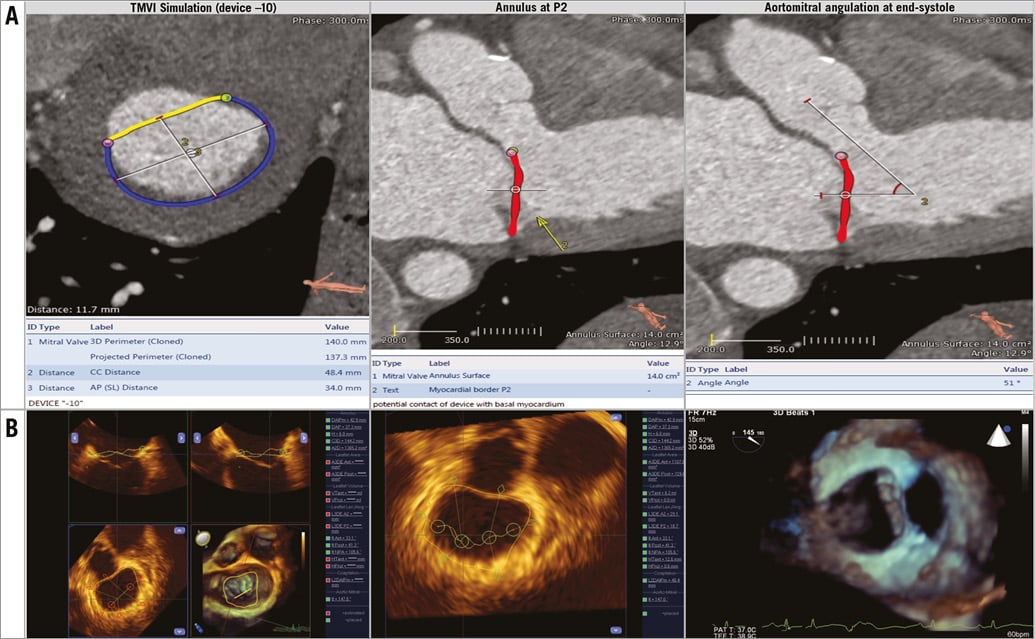
Figure 2. Valve sizing. A) Multislice computed tomography imaging. B) Three-dimensional transoesophageal echocardiography.
Clinical results
The first devices were implanted surgically by Lutter and colleagues after extensive preclinical work on similar devices by this group. The first reported cases of permanent human implantation of the Tendyne system were performed in the autumn of 2014 at the Royal Brompton Hospital in London . Since then, this single centre has treated five patients under a compassionate use protocol. At 30 days, mortality was zero. Moreover, there were no strokes, vascular complications or bleeding events in the first 30 days after implantation. All but patient 4 reported early symptomatic benefit and were discharged independent in activities of daily living back to their own home after a mean length of hospital stay of 10 days. The fourth patient, who had previously undergone mitral valve repair with a 38 mm Carpentier-Edwards Physio II ring (Edwards Lifesciences, Irvine, CA, USA), developed periprocedural LV outflow tract obstruction (LVOTO). This was a very complex and dynamic phenomenon but occasionally the LVOT pressure drop was close to 100 mmHg. There clearly was a degree of fixed narrowing but the main problem appeared to be due to systolic anterior motion of a long unsupported anterior mitral valve leaflet, i.e., rather than simply a fixed narrowing induced by the outer stent. Five days after the TMVI procedure, a 22 mm CP stent mounted on a 20 mm balloon was placed in the LVOT to alleviate the dynamic LVOTO by pinning the anterior leaflet between the CP Stent™ (pfm medical, Cologne, Germany) and the outer stent of the Tendyne device. This resolved the dynamic nature of the LVOTO with a dramatic clinical improvement. The patient had a prolonged hospital stay and was discharged home after 65 days, independent in activities of daily living (and remains well >1 year after the procedure with a peak LVOT gradient of 25-30 mmHg).
At 30 days, transthoracic echocardiography showed no patient had transvalvular mitral regurgitation and none had a significantly raised transmitral gradient. One patient had developed a trivial paravalvular leak, which was associated with biochemical haemolysis and which required a transfusion of 2 units of packed red cells on a single occasion. No patient developed new LVOTO.
At six months after TMVI, all patients were alive, living at home and independent in activities of daily living. All reported continued symptomatic benefit with reduction in NYHA class. All valves were stable with no migration and no new paravalvular leak. There was significant reduction in the severity of tricuspid regurgitation and pulmonary hypertension. The trivial leak noted in one patient at 30 days was unchanged at six months, with a reduction in the indices of haemolysis. One concern with the Tendyne device was what effect a reduction in tether tension secondary to left ventricular remodelling over time might have. These six-month data would suggest that, if tension does reduce, it is not accompanied by device instability or new late paravalvular leak. This is presumably due to tissue ingrowth and healing.
The 30-day outcomes of the first 23 patients in the Global Feasibility Study of the Tendyne Mitral Valve System were presented at EuroPCR, 2016. These data demonstrated promising results and will be the subject of a future manuscript. They confirmed the findings in the compassionate use patients that this system was effective at eliminating mitral regurgitation in the vast majority of patients, with trivial residual mitral regurgitation in only a small proportion. The screen pass rate for patients submitted for consideration to participate in this study was significantly greater in patients with secondary compared with primary mitral regurgitation owing to the latter group tending to have smaller ventricles with an increased potential for LVOTO. A modified valve design is being developed in order to increase the number of patients who potentially can be treated with the Tendyne system. This iteration is designed to have a lower profile in the left ventricle and is thus anticipated to create a larger “neo-LVOT” after implantation. This should enable the Tendyne system to be implanted in patients who would currently be at risk of LVOTO.
A second modification is to the delivery system. The original delivery system had a hand squeeze and zip mechanism for valve deployment and a separate sheath/dilator. The second-generation delivery system has an integrated sheath with removable balloon dilator with wheel rotation for valve deployment. A new pad positioning tool will also have wheel mechanisms for tensioning, pin drive, and pad release.
Conclusion
The early experience with the Tendyne system is promising. Stabilisation by means of an apical tether is a novel way to secure fixation and minimise the risk of device migration. The Tendyne device is very effective at eliminating mitral regurgitation and would appear to be stable up to at least six months. There is an ongoing pipeline of device and delivery system development. The unique design features of this device offer the potential for it to be useful across a wide range of mitral valve pathologies.
Conflict of interest statement
N. Moat has been a consultant to Tendyne. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Insertion of a balloon-tipped catheter to exclude entrapment in the mitral subvalvar apparatus.
Moving image 2. Extrusion of the implant device within the left atrium through a 34 Fr sheath.
Moving image 3. The Tendyne device is pulled into the mitral annulus to complete deployment.
Supplementary data
To read the full content of this article, please download the PDF.
Insertion of a balloon-tipped catheter to exclude entrapment in the mitral subvalvar apparatus.
Extrusion of the implant device within the left atrium through a 34 Fr sheath.
The Tendyne device is pulled into the mitral annulus to complete deployment.

