Introduction
Treatment of mitral regurgitation by transcatheter mitral valve replacement (TMVR) is feasible with over a hundred completed procedures worldwide as part of numerous clinical feasibility studies led by multiple manufacturers. Mitral valve anatomy is dynamic and variable throughout the cardiac cycle1, presenting a unique challenge for TMVR devices to ensure sealing and fixation whilst providing good haemodynamic performance and procedural feasibility. Herein, we describe the technical and product features of the Intrepid™ TMVR system (Medtronic Inc., Minneapolis, MN, USA) which is under current investigation in a worldwide multicentre clinical study.
Device description
The Intrepid TMVR system consists of a bioprosthesis and transapical delivery system. The bioprosthesis (Figure 1, Moving image 1) is a trileaflet bovine pericardial valve contained in a self-expanding nitinol frame which has a unique dual structure design consisting of a circular inner stent to house the valve and a conformable outer fixation ring to engage the mitral annular anatomy. The outer fixation ring is designed to accommodate the dynamic variability of the native mitral annulus while isolating the inner valve assembly throughout the cardiac cycle. A flexible brim is attached to the atrial end of the fixation ring which facilitates imaging during the procedure.
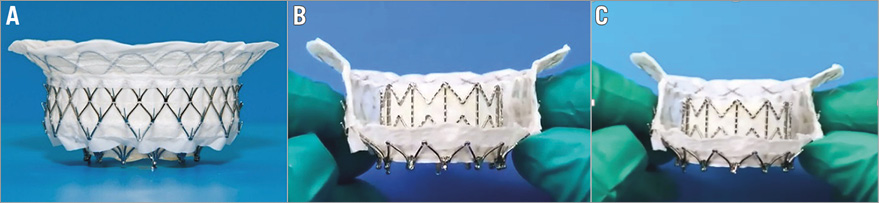
Figure 1. Device images. A) The Intrepid TMVR prosthesis, B) with cut-outs to demonstrate the innovative dual stent design, and C) overall flexibility which allows the device to conform to the shape of the native mitral annulus.
The bioprosthesis is built around a 27 mm inner valve structure with an effective orifice area (EOA) of 2.4 cm2 and is being investigated in 43 mm, 46 mm, and 50 mm outer diameters. Fixation and sealing are achieved through a combination of design features: A) the outer fixation ring is larger in circumference than the native mitral valve annulus with varying degrees of radial stiffness along its axial length, B) the atrial portion of the outer fixation ring is flexible where the frame and native annulus engage allowing conformation to the native annulus – in contrast, the ventricular portion is stiffer and resists compression, C) the flexible atrial portion deflects inward to allow annular alignment while the stiff ventricular mid-section resists compression and maintains its shape, producing a final “champagne cork-like” conformation (narrow neck and wider body) to resist migration under systolic pressure (Figure 2). Three circumferential rings of frictional elements further help fixation. The prosthesis is designed to preserve the native leaflets and chordae and leverage them to seal around the device. The outer and inner stent frames are covered by a polyester fabric skirt to assist in preventing paraprosthetic leaks and facilitate tissue ingrowth for long-term fixation and sealing. The prosthesis has minimal protrusion downstream of the annulus to help maintain patency of the left ventricular outflow tract.
With its unique dual structure design, the Intrepid bioprosthesis separates haemodynamic function of the inner valve assembly from sealing and fixation provided by the outer frame. The outer fixation ring conforms to the native mitral anatomy throughout the cardiac cycle while the inner valve assembly remains circular; maintaining the circular shape is anticipated to provide durable and consistent haemodynamic characteristics. The brim of the device is designed to accommodate the variability and features of the atrial anatomy as demonstrated in Figure 2 and, over time, provide additional surface area to promote tissue ingrowth.
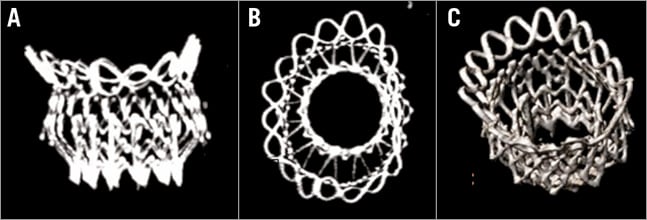
Figure 2. X-ray computed tomography images from a human implantation of the Intrepid prosthesis. A) Septal-lateral view, B) en face view and C) oblique view.
Integration of the implant within the heart is illustrated in Figure 3. Healing of the atrial space between the stents follows a characteristic sequence of events which is common to virtually all cardiac devices2. An initial layer of fibrin deposition (resulting from acute inflammation) resolves, leading to endothelial encapsulation as the surface thrombus organises to provide a protective adherent coating. Ultimately, a pannus layer between the stents forms a new atrial floor.
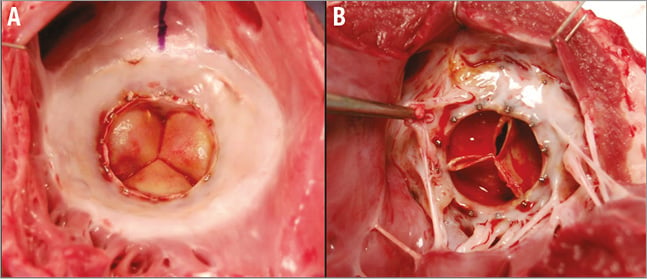
Figure 3. Integration of the implant within a porcine preclinical model. Healing response observed on the A) atrial and B) ventricular surfaces three months following Intrepid prosthesis implantation.
Delivery system
The Intrepid TMVR delivery system is currently designed for transapical access only and consists of an apical introducer sheath (with dilator) and a hydraulically actuated delivery catheter. The prosthesis is compressed to 33 Fr prior to loading within the delivery capsule then advanced via the apical sheath into the left ventricle and across the mitral valve. Under echocardiographic guidance, the capsule is retracted hydraulically using a standard inflation device to gradually deploy and release the self-expanding prosthesis within the native mitral valve (Figure 4). The hydraulic actuation system assists with controlled expansion and deployment of the prosthesis. Importantly, the system does not require rotational alignment, tethering or capture of native leaflets before or during device deployment.
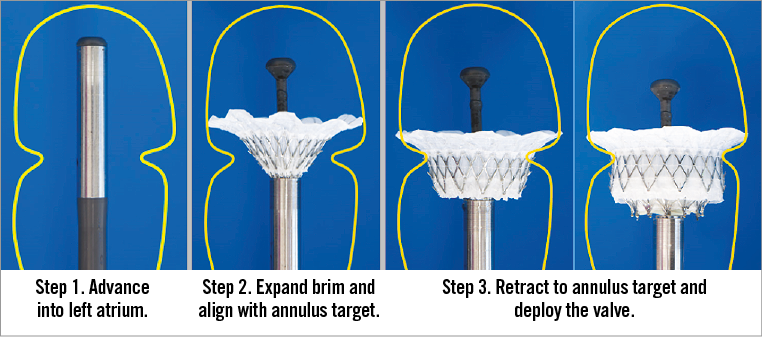
Figure 4. The delivery sequence for implantation of the Intrepid bioprosthesis.
The Intrepid TMVR system has undergone extensive in vivo and in vitro testing as mandated by international guidelines3. The Intrepid TMVR system meets its product performance and design specifications with durability data out to 200 million cycles for the valve and 600 million cycles for the implant structures. Early clinical evaluation of the system is underway in an on-going international multicentre clinical study. The combination of a novel straightforward delivery system and a uniquely designed bioprosthesis that does not require rotational alignment, tethering or capture of the native mitral leaflets for fixation lends itself to what could be a promising advance for treating mitral valve disease.
Conflict of interest statement
I.T. Meredith is a consultant to Boston Scientific, Elixir and Medtronic. V. Bapat is a consultant for Edwards Lifesciences, Medtronic and Boston Scientific. J. Morriss and M. McLean are employees of Medtronic, Inc. B. Prendergast has no conflicts of interest to declare.
Supplementary data
Moving image 1. Demonstration of the fixation ring and inner valve of the Intrepid TMVR prosthesis. The outer fixation ring is designed to accommodate the dynamic variability of the native mitral annulus while isolating the inner valve assembly throughout the cardiac cycle.
Supplementary data
To read the full content of this article, please download the PDF.
Demonstration of the fixation ring and inner valve of the Intrepid TMVR prosthesis. The outer fixation ring is designed to accommodate the dynamic variability of the native mitral annulus while isolating the inner valve assembly throughout the cardiac cycle.

