Device description
Name and manufacturer: Edwards FORTIS transcatheter mitral valve (TMV); Edwards Lifesciences, Irvine, CA, USA.
Approval status: Approved for use under three protocols: special access in Canada, feasibility study in Europe, and feasibility study in the USA. Not approved for sale in any country.
Platform: Cloth-covered, self-expanding nitinol stent with three bovine pericardial leaflets.
Specific design: The FORTIS TMV (Figure 1) consists of: 1) a central valve body which is circular and cylindrical measuring 29 mm in diameter, having three pericardial leaflets, treated with proprietary anticalcification treatment, sutured within the nitinol stent; 2) paddles which are located in the outflow of the central valve body allowing capture of mitral leaflets to anchor the FORTIS TMV; 3) an atrial flange which is at the inflow of the central valve body and made up of multiple nitinol struts covered with cloth. Two arms are flexible to prevent aortic sinus impingement. The flange rests on the base of the left atrium and allows tissue endothelialisation.
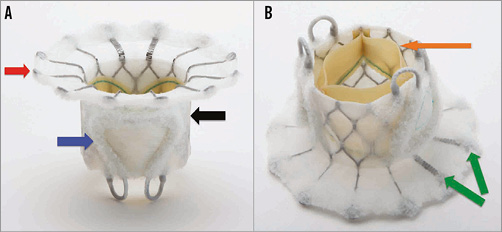
Figure 1. FORTIS transcatheter mitral valve. A) Side profile of the FORTIS valve highlighting the atrial flange (red arrow), body of the FORTIS valve (black arrow) and one of the two paddles (blue arrow). B) Side profile of the FORTIS valve highlighting the bovine pericardial leaflets (orange arrow) and the flexible struts, which align with the A2 segment of the mitral valve.
Delivery system: The FORTIS TMV is implanted through a transapical (TA) approach using a dedicated sheathless delivery system. The delivery system is 42 Fr in outer diameter. The sheath has a nose cone, which allows easy insertion and harbours the atrial flange. The main shaft is made of a harness and two sheaths which hold the central valve body and the flange. The main handle has two knobs which control the movements of the inner and outer sheaths which cover and uncover parts of the FORTIS device. A knob at the end of the handle controls the paddles. The multiple levels of control allow stepwise release of the valve and also allow recapturability and repositionability to a certain point during deployment. The delivery system has a central lumen for a guidewire and has multiple radiopaque markers which assist positioning and deployment of the device under fluoroscopy and echocardiography.
Device sizes: The only available device measures 29 mm in diameter.
Procedural details (Figure 2, Moving image 1, Moving image 2)
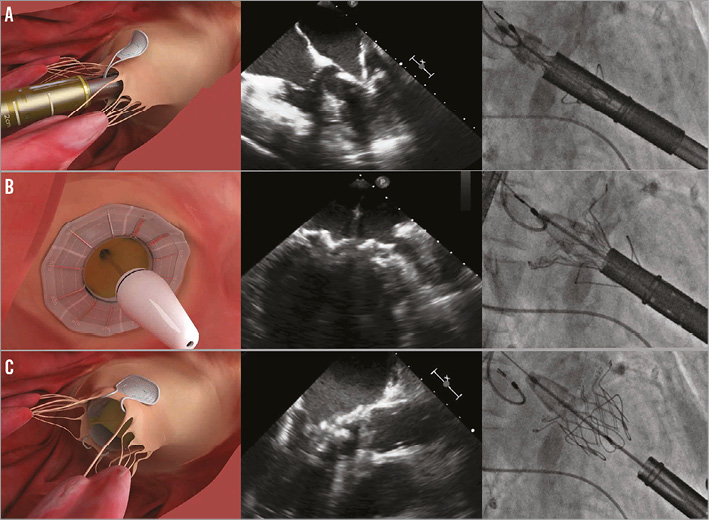
Figure 2. Important steps in FORTIS valve implantation illustrated with a picture, TEE image and fluoroscopic image. A) Leaflet capture. The paddles are seen outside both the leaflets. Closing will result in trapping the leaflets between the paddles and the valve body. B) Release of the atrial flange. Once leaflet capture is confirmed the atrial flange is released by pushing the nose cone forward. C) Valve deployment. Once the paddles are closed over the body to anchor the device securely, the valve is released by unsheathing the ventricular portion.
The procedure is performed under general anaesthesia with transoesophageal echocardiography (TEE) and fluoroscopy guidance1. Access to the apex is similar to the transapical approach for TAVI. A regular 0.035 inch wire is placed in the left atrium. The wire must be free from chordal entanglement and exchanged for an Amplatz Super Stiff™ guidewire (Boston Scientific, Marlborough, MA, USA). The apex is sequentially dilated prior to introduction of the delivery system. The delivery system is passed until the nose cone is at the level of the mitral annulus. The paddles are unsheathed when they are perpendicular to the mitral coaptation line and opened up to 45 degrees to capture the A2-P2 mitral leaflets. Once this is confirmed, the device continues to be unsheathed into the native mitral annulus. Final checks are made to confirm optimal positioning and native mitral leaflet capture prior to releasing of the atrial flange. Up to this stage, the device is completely recapturable and repositionable. Once the atrial flange is released, the paddles are closed and the device is completely released. Once TEE has confirmed the stability and function of the valve, the apical access site is closed and the chest is closed in the routine manner.
Clinical data
Twenty patients who are inoperable or high risk for conventional mitral valve surgery and unsuitable for current transcatheter alternatives have had the FORTIS TMV implant either on compassionate grounds or under feasibility protocols. The results of 13 of the 20 patients are available due to ongoing feasibility trial protocol in the USA. Baseline characteristics are summarised in Table 1.
Procedural success occurred in 10 patients (76.9%). Procedural time was 48±19 minutes. Two patients were converted to surgery, one because of malposition of the device and another because of chordal entanglement of the balloon before device implant (17.4%). One patient had partial migration of the device because of incomplete posterior leaflet capture and died on day four. In-hospital mortality rate was 30.8% (4/13). Echocardiography at the time of discharge (9/13 patients) demonstrated excellent function with mean transmitral gradients of 3±1 mmHg, no or trace mitral regurgitation (MR) in eight patients and mild in one. At 30 days, the mortality rate was 38.5% (5/13). One patient who was discharged on warfarin and aspirin was readmitted with chest pain and signs of sepsis. Echo confirmed increased gradients across the valve. The patient died on day 15 due to suspected valve thrombosis/infective endocarditis. Of the survivors beyond 30 days (8/13), there was one death at day 76 due to heart failure and related complications. The device was functioning satisfactorily.
Ongoing studies
FORTIS implants under all protocols were voluntarily put on hold because of evidence of thrombosis in two additional patients. Details of the root cause analysis will be available soon and early signs are that the study will restart with a stringent anticoagulation protocol.
Unique features
Self-expanding circular valve to reduce the stress of leaflets during closure and maximise forward blood flow. Unique anchoring mechanism utilising paddles, which help capture the mitral leaflets. Cloth cover across the device to promote tissue endothelialisation. Multi-control delivery system allows stepwise release and recapturability of the device.
Potential improvements
Improved patient screening for suitability of the device not only for anatomical fit but also for apical access and predicted functional result. Patients who have end-stage mitral valve disease with significant left ventricular geometry distortion might not benefit from the device. Refining imaging during the procedure to confirm leaflet capture, which is the most important step in the implantation of the device.
Conflict of interest statement
V. Bapat is a consultant for Edwards Lifesciences, Boston Scientific and Sorin. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Animation demonstrating stent design and important steps in the FORTIS TMV implantation.
Moving image 2. Short clip of the procedure demonstrating key steps in the deployment of the FORTIS TMV.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Animation demonstrating stent design and important steps in the FORTIS TMV implantation.
Moving image 2. Short clip of the procedure demonstrating key steps in the deployment of the FORTIS TMV.

