Description - technical specifications
The main support structure of the Portico™ valve (St. Jude Medical, Inc., St. Paul, MN, USA) is a radiopaque, self-expanding nitinol stent (Figure 1). The stent has a non-flared annulus design with large open cells. The trileaflet bovine pericardial valve has a contoured design to improve haemodynamic performance and durability. A porcine pericardial sealing cuff at the annular level of the stent constitutes the sealing zone. All tissues are processed using the Linx™ anti-calcification technology (St. Jude Medical, Inc.).
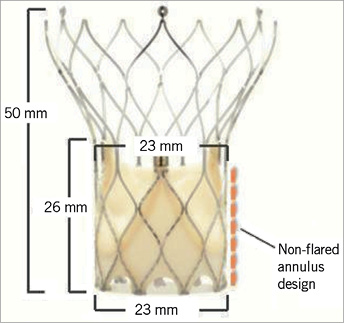
Figure 1. Design of the Portico™ 23 mm valve.
The Portico™ valve is designed to be implanted at the annular level extending no more than 5 mm into the left ventricular outflow tract, potentially minimising conduction defects. The large aortic cells and the annular valve position facilitate engagement of the coronary ostia post implantation (Figure 2). A 23 mm valve is currently available and additional sizes of 25, 27 and 29 mm are anticipated. Criteria for implantation of the Portico™ 23 mm valve are listed in Table 1.
Figure 2. Coronary angiography of the left system post implant.
The transapical delivery system (Figure 3) is a 24 Fr (outer diameter) sheathless system that deploys the annulus section of the valve first. The position of a partially deployed valve can be evaluated and, if needed, resheathing, repositioning or retrieval can be performed (Figure 4). The distal end of the delivery system is designed with a soft, tapered, radiopaque tip with a pre-curved section to assist in traversing the aortic arch. A handle attached at the proximal end of the delivery system is used to load, deploy and resheath the valve by operation of a single thumbwheel. Dedicated loading tools allow fast and easy preparation and loading of the valve. The valve is prepared and loaded onto the delivery system at room temperature.
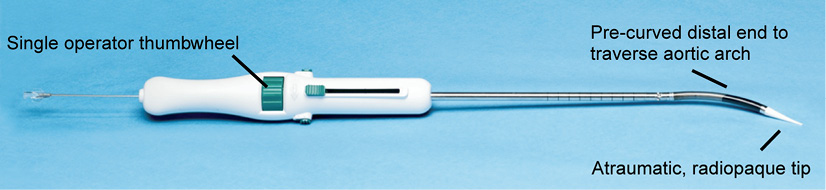
Figure 3. Dedicated Portico™ transapical delivery system. The loaded delivery system does not require the use of an introducer sheath.
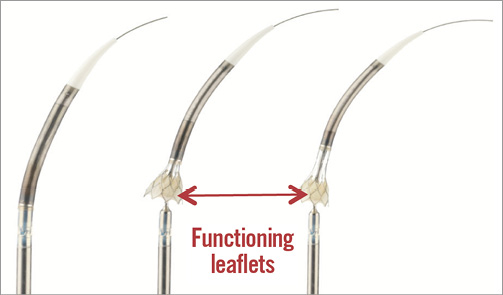
Figure 4. Transapical delivery system with annulus first deployment. Left: the compressed valve is completely sheathed within the delivery system. Middle: partial deployment of the annular section of the valve. At this point the valve begins to function. Right: the stent is unsheathed up to 80% and can be completely resheathed at this point to return to the configuration shown in the left panel, and repositioned or retrieved from the patient, if necessary.
Valve implantation (transapical approach)
The 23 mm Portico™ valve is designed to accommodate annulus diameters between 19 mm and 21 mm. Implantation of the valve is performed using transoesophageal echocardiography (TEE) and fluoroscopy. Left ventricular (LV) apical access is obtained by a standard left mini anterior thoracotomy, followed by placement of apical haemostatic sutures. Unfractionated heparin is given to achieve an activated clotting time >300 seconds. During preparation of the apical site, the valve is loaded onto the transapical delivery system using a dedicated valve loading system. The LV apex is punctured and the aortic valve (AV) crossed with a soft J guidewire, over which a 7 Fr sheath is placed across the AV. Wire exchange with a 0.035” Amplatz Super Stiff (Boston Scientific, Natick, MA, USA) is performed and the wire is positioned across the aortic arch into the descending aorta. Aortic valvuloplasty is performed through a 14 Fr sheath.
The transapical delivery system is inserted directly through the apex with no introducer sheath required. The delivery catheter is slowly advanced across the aortic valve until the radiopaque marker is aligned with the annulus of the native aortic valve (Figure 5A). Aortography and TEE are employed to verify that the most distal valve segment is approximately 3-5 mm below the annulus and aligned with the annular plane.
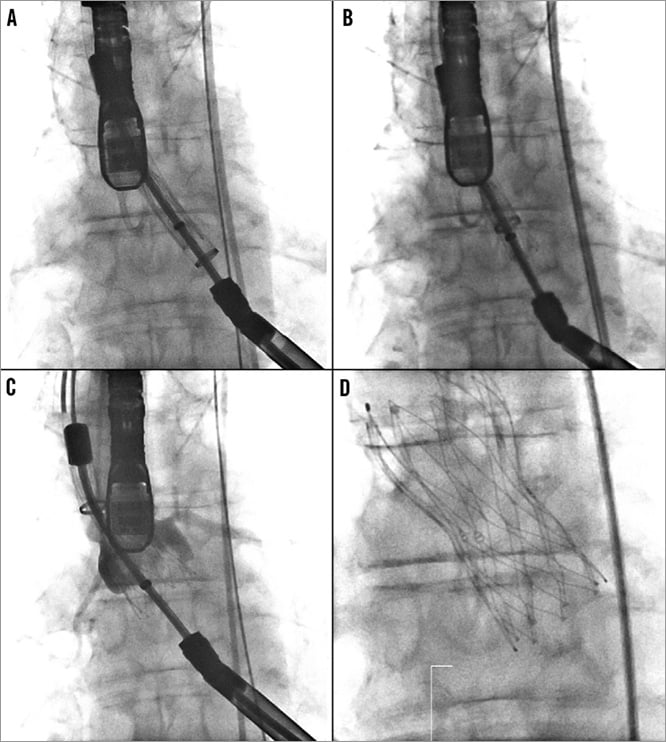
Figure 5. A) Portico™ valve is advanced across the aortic valve aligning the radiopaque marker with the native aortic annulus. B) Gradual valve deployment without rapid pacing. C) Delivery system unsheathed at 80%. Valve leaflets are fully functioning. Valve can be fully resheathed to reposition or retrieve. D) Full deployment of the Portico™ valve.
Gradual valve deployment, without rapid pacing, is achieved by rotating the thumbwheel on the handle (Figure 5B). While maintaining valve position, the stent can be unsheathed up to 80%. The valve is functional upon deployment of the annular part of the stent (Figure 5C). At any point leading up to 80% deployment, the valve can easily be partially or fully resheathed, repositioned or retrieved from the patient. Optimally, the device is implanted at a depth of 3 mm to 6 mm. If re-crossing the annulus is required, then complete resheathing can be performed and the valve redeployed. Resheathing can be accomplished by reversing the direction of the thumbwheel on the delivery system handle.
After assessing placement accuracy, continual rotation of the thumbwheel allows full deployment of the device and release from the delivery system (Figure 5D). Prior to withdrawing the delivery catheter, release of all three retention tabs from the delivery system should be confirmed, preferably by two different fluoroscopy views. Following release from the delivery system valve functioning is assessed by standard methods.
Clinical experience
The 23 mm Portico™ valve results using the transfemoral delivery system have been collected from the first-in-human study. This study was carried out in Canada (St Paul’s, Vancouver, and Quebec Heart and Lung Institute, Quebec City, Canada) and Europe (Royal Victoria Hospital, Belfast, United Kingdom)1,2. The early clinical experience was good and substantial haemodynamic improvements were observed post procedure and at 30-day follow-up.
The first-in-human transapical cases were performed using the transapical system at St. Paul’s Hospital, Vancouver, and the Quebec Heart and Lung Institute, Quebec City, Canada. A total of seven cases were successfully performed. Procedure successes were 100% by VARC definition, without valve malpositioning or embolisation. A single procedural mortality was encountered due to a delayed haemorrhage from the chest wall. No major strokes or myocardial infarctions were reported at 30 days. Paravalvular regurgitation was mild or trivial in all patients. All other patients had significant functional improvement at clinical follow-up.
Conclusions
First-in-human experience is underway in Canada with the St. Jude Medical Portico™ transapical delivery system. Clinical data from the first-in-human experience with the Portico™ TAVI transapical system suggest that it is a safe and user-friendly system for transcatheter aortic valve implantation. The self-expandable Portico™ valve system incorporates novel capabilities, such as the ability to be fully resheathable, repositionable and retrievable. This may be helpful in achieving optimal valve positioning and placement, thus mitigating complications such as valve malpositioning, embolisation and severe paravalvular leakage. Furthermore, this self-expandable system does not require ventricular pacing during deployment, thus minimising haemodynamic decompensation. In our initial clinical experience, substantial haemodynamic improvements are accomplished without major cardiovascular complications.
Additional valve sizes and delivery systems for subclavian and transaortic access routes are anticipated. Further procedural experience with current and anticipated device sizes and access sites, as well as longer-term outcome data from larger patient cohorts, are required for full evaluation of this novel TAVI system.
Conflict of interest statement
A. Cheung is a consultant for St. Jude Medical, Edwards Lifesciences, HeartWare Inc., Abiomed Inc., Entourage Medical Technologies Inc., Neovasc Inc., and Kardium Inc. R. Makkar is a consultant/national PI/SAB for St. Jude Medical, a consultant for and equity holder in Entourage Medical Technologies, and a consultant/site PI for Edwards Lifesciences. G.P. Fontana is a consultant/national PI/SAB for St. Jude Medical, a consultant for and equity holder in Entourage Medical Technologies, a consultant/site PI for Medtronic, a consultant/site PI and on the speaker’s bureau for Sorin Medical, and a consultant for Edwards Lifesciences.

