Abstract
Aims: Transcatheter aortic valve implantation (TAVI) has become a viable option for selected high-risk patients with severe and symptomatic aortic stenosis. First- and second-generation TAVI devices are either self- or balloon-expandable, and are often not repositionable or not fully retrievable, leading to suboptimal positioning in some cases. This may result in paravalvular regurgitation, AV conduction delay, or compromise of coronary perfusion. A broader application of TAVI requires advances in both valve and delivery systems. Therefore, in order to facilitate accurate positioning, to minimise paravalvular leakage, possibly to reduce the risk of AV conduction delay, and possibly to be able to abort the procedure, a “next-generation” TAVI system has been developed which is repositionable and retrievable, the TRINITY heart valve system.
Methods and results: The TRINITY heart valve system was implanted in a first-in-human study using the transapical approach to demonstrate feasibility and procedural success. All endpoints were adjudicated according to VARC definitions at seven and 30 days. The TRINITY heart valve system was implanted in a 74-year-old patient with severe symptomatic aortic valve stenosis. In this case, repositioning of the TRINITY resulted in optimal position without paravalvular leakage and with perfect function.
Conclusions: The TRINITY heart valve is a repositionable and retrievable TAVI system. Both the implantation result and short-term clinical and haemodynamic outcome were excellent.
Introduction
Transcatheter aortic valve implantation (TAVI) has been proven to be a valid alternative for elderly patients with severe and symptomatic aortic stenosis1. In clinical studies, TAVI has proven to be feasible, effective and safe with significantly reduced morbidity and mortality when compared to medical therapy in patients with prohibitive risk for surgery2. Furthermore, the use of TAVI has been shown to be non-inferior to conventional surgical aortic valve implantation in high-risk patients3. However, at the current stage, there are limitations and procedure-related complications which include paravalvular leakage, AV conduction delay, and compromise of coronary perfusion4,5. Therefore, a next-generation TAVI system was developed, which is repositionable and retrievable in order to facilitate accurate positioning, to minimise paravalvular leakage, to reduce the risk of AV conduction delay, and possibly to be able to abort the procedure.
Characteristics of the TRINITY heart valve system
The TRINITY heart valve (Transcatheter Technologies GmbH, Regensburg, Germany) is a self-expandable valve that consists of three glutaraldehyde-treated bovine pericardial tissue leaflets attached to a nitinol frame (Figure 1). The valve is folded to a minimal diameter by strings attached to an upper and lower crown (Figure 2). A dedicated technology to protect the pericardial leaflets during crimping of the prosthesis has been developed (Zero Pressure Crimping™; Transcatheter Technologies GmbH), aiming to increase valve durability. The lower crown is positioned in a supra-annular fashion while the upper crown helps to centre the valve and to maintain the optimal valve geometry at the level of coaptation. The height of the prosthesis is 23 mm. During folding and expansion by means of the attached strings, the TRINITY valve displays a stable frame height without any foreshortening. In order to minimise paravalvular regurgitation, a pericardial cuff is placed along the lower crown for additional sealing. The supra-annular position of the prosthesis, with the lower crown not interfering with the left ventricular outflow tract, aims at minimising the risk of atrioventricular conduction disturbances.
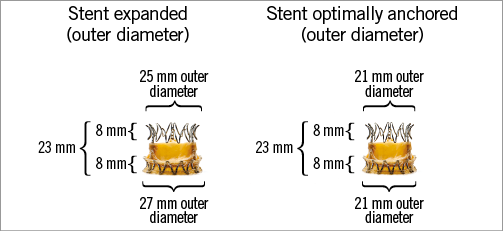
Figure 1. The TRINITY heart valve is a self-expandable valve prosthesis that consists of three bovine pericardial tissue leaflets attached to a nitinol frame.
The TRINITY heart valve prosthesis is available in a 20 mm size for an annulus size between 19 and 21 mm. Additional prosthesis sizes for treating larger annulus sizes are under development.
The TRINITY valve comes premounted on a catheter tip that is connected to the delivery catheter in the operating theatre just before implantation. By a quarter turn of the wheeled handle, the prosthesis can be unfolded and refolded multiple times. In this way, the system allows for precise stepwise positioning of the TRINITY valve with the possibility to reposition and to retrieve the valve prosthesis, if ever the procedure needs to be aborted (Figure 2).
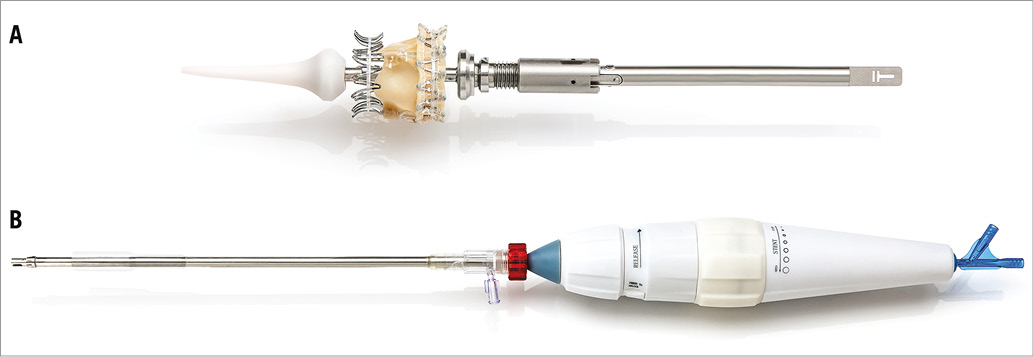
Figure 2. Implantation set of the TRINITY heart valve consisting of two parts. A) The TRINITY heart valve is premounted on the catheter tip using the Zero Pressure Crimping™ leaflet protection. B) The catheter tip with the premounted valve is connected to the delivery catheter just before implantation.
First-in-human experience
A first-in-human experience with the 20 mm TRINITY aortic valve system was performed successfully in a 74-year-old patient suffering from severe, symptomatic aortic stenosis. The diagnostic procedures included transthoracic and transoesophageal echocardiography, and computed tomography. The patient had a body mass index of 35.1 kg/m², suffered from dyspnoea NYHA Class III, had a history of arterial hypertension and was free of coronary artery disease. In blood evaluation, she had normal kidney function with a creatinine level of 0.9 mg/dL. On echocardiography, the ejection fraction was 59%, the peak and mean pressure gradients were 120 and 58 mmHg, respectively, and the aortic valve area was 0.6 cm². There was minimal aortic regurgitation with a PHT of 4,400 ms. The annulus size was measured to 20 mm. Pulmonary artery pressure was 17 mmHg above central venous pressure, with minimal mitral regurgitation. Coronary angiography was normal. Computed tomography showed the descending aorta having severe calcification in the height of the bifurcation, such that a transfemoral approach was not possible. When calculating the risk scores, the logistic EuroSCORE I was 4.5%, and the STS score 2.95%.
Considering the diagnostic procedures together, this symptomatic patient was found to be suitable for the TRINITY device. The implantation of the TAVI device was performed in Caracas, Venezuela, and was approved by the local ethics committee.
At the first positioning, the valve prosthesis was anchored slightly too low, leaving a relevant paravalvular leakage. Repositioning of the TRINITY valve was performed and the valve was anchored approximately 2 mm more in the direction of the aorta. After repositioning, re-evaluation of the device performance revealed a markedly improved result with no paravalvular leakage. The intraprocedural invasive peak-to-peak gradient was excellent with 5-8 mmHg.
At 30-day follow-up, the patient had markedly improved clinically to NYHA functional Class I. The effective orifice area had improved from 0.3 cm2 to 1.4 cm2, and the mean transvalvular gradient was significantly reduced from 135 to 20 mmHg. There was no prosthesis/patient mismatch, as indicated by an indexed effective orifice area of 1.2±0.2 cm2/m2, which is considered to be absent at >0.7 cm2/m2 for a BMI ≥30 kg/m2.6 At 30-day follow-up, no paravalvular leakage and no AV block requiring pacemaker implantation were observed.
Valve implantation process (transapical approach)
After standard surgical preparation of the left ventricular apex (using an approximately 4 cm submammary incision), balloon aortic valvuloplasty is performed with a commercially available valvuloplasty balloon. Subsequently, the folded TRINITY valve prosthesis is advanced through a 31 Fr sheath into the left ventricle and further into the aortic root (Figure 3A). The sheath protecting the crimped valve during insertion is retracted while the prosthesis still remains folded. The position is controlled via fluoroscopy. During beating heart without rapid left ventricular pacing, the delivery wheel on the handle is turned by one quarter, expanding the valve (Figure 3B). The prosthesis can be fully anchored before its position is checked, and valvular function is evaluated by injection of contrast medium into the ascending aorta (Figure 3C) or by transoesophageal echocardiography. In the case of a suboptimal position, the prosthesis can be rapidly and fully folded by turning the wheel in the opposite direction. The device can be repositioned several times until it reaches a satisfactory position. Once the final position is achieved, the attached strings are released and retracted. It is of note that, during final valve release, the geometry of the prosthesis does not change anymore (Figure 3B, Figure 3C). An animation of the implantation process can be seen on the company’s website at http://www.transcatheter-technologies.com/home.html.
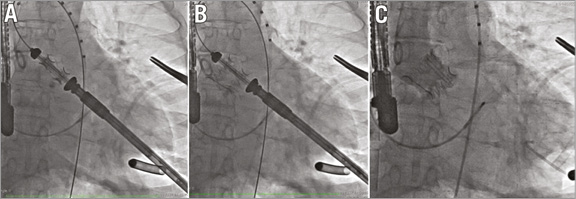
Figure 3. Delivery and deployment of the TRINITY heart valve system (courtesy of C. Hengstenberg, Deutsches Herzzentrum München, and Dr José Condado, Hospital Miguel Perez Carreno, Caracas, Venezuela). A) TRINITY heart valve system crossing the native annulus. B) Initial flare and positioning of TRINITY heart valve system in preparation for deployment. C) Final deployment and position of the TRINITY heart valve system.
True repositionability
There are several nitinol-based self-expanding TAVI devices claiming repositionability during the implantation process. In general, self-expanding valve prostheses are made of nitinol and are crimped and mostly held in place by a sheath around the folded frame. For deployment, the sheath is retracted, the frame unfolds partially, and anchors consecutively. Therefore, during deployment, the frame does not have its final shape and major changes in frame geometry may occur upon final release. This change in frame geometry makes it difficult to predict the final result for both position and function.
The major advantage of the TRINITY valve as compared to other transapical TAVI valves is its full repositionability. This improvement, together with further advances in its construction, gives it very accurate positioning and a very low degree of aortic regurgitation. When the valved frame is entirely deployed, expanded and anchored, it is still connected to the delivery system and can be refolded easily. The functioning of the prosthesis can be fully evaluated before the operator decides to continue the implantation procedure. Once the result is considered to be satisfactory, the entirely deployed and anchored valve is disconnected from the delivery catheter without further changes of the frame shape, geometrical form, and function.
Supra-annular implantation
To achieve sufficient anchoring, most TAVI prostheses rely on an anchoring zone, reaching from the left ventricular outflow tract along to the aortic root distally and beyond the sinotubular junction. Anchoring in the left ventricular outflow tract may cause compression of the atrioventricular conduction system with a high risk for conduction disturbances leading to pacemaker implantations. Due to the design of the TRINITY aortic valve, the lower crown is implanted at the lowest points of the leaflet attachment line, anchoring at the level of the inter-leaflet triangles. Accordingly, the TRINITY frame reaches into the left ventricular outflow tract, does not compress myocardial structures and does not create pressure on the conduction system in the left ventricular outflow tract. Therefore, the TRINITY valve system may significantly reduce the incidence of conduction disturbances and the need for permanent pacemaker implantation.
Conclusions
The novel self-expandable TRINITY valve prosthesis is a fully repositionable valve system. The TRINITY valve prosthesis can be safely implanted in a stepwise fashion, and expanded and folded without foreshortening, allowing for controlled deployment. After complete expansion and anchoring, the valve function can be fully evaluated, still allowing for true repositioning capabilities or complete retrieval if needed. The supra-annular anchoring of the prosthesis, with the lower crown not interfering with the left ventricular outflow tract, aims at minimising the risk of atrioventricular conduction disturbances. A pericardial cuff along the lower crown of the valved stent provides exceptional sealing. First-in-human clinical experience with the TRINITY aortic valve system is very promising. Larger case series with longer clinical follow-up are required to demonstrate the safety and efficacy of this transcatheter valve system.
| Impact on daily practice Transcatheter aortic valve implantation (TAVI) has become a viable option for selected high-risk patients with severe and symptomatic aortic stenosis. The TRINITY heart valve system was implanted in a first-in-human study using the transapical approach to demonstrate feasibility and procedural success. Both the implantation result and short-term clinical and haemodynamic outcome were excellent. |
Conflict of interest statement
C. Hengstenberg is a consultant for Transcatheter Technologies GmbH, Regensburg, Germany. The other authors have no conflicts of interest to declare.

