
Abstract
Aims: While TAVI is the treatment of choice in patients with aortic stenosis considered inoperable or at high risk, interventional replacement of the mitral valve is still in the preclinical or early clinical phase. Our aim was to report on the first transcatheter double valve replacement into native valves from a transapical access.
Methods and results: A 67-year-old, highly symptomatic female patient considered inoperable due to severe calcification of the mitral annulus and comorbidities was scheduled for transcatheter double valve replacement by the local Heart Team. Preoperative planning was carried out by multiplanar reconstruction from cardiac CT. Through a transapical access, the mitral valve was replaced first by an inverted 29 mm Edwards SAPIEN 3 prosthesis, then the aortic valve by a 23 mm SAPIEN 3, both during rapid pacing. Both prostheses revealed excellent function in angiography and echocardiography. The patient was extubated early after surgery and transferred to the normal ward the following day. After five months, she exhibited signs of cardiac failure again. Migration of the mitral prosthesis was detected, and the mitral valve was replaced surgically.
Conclusions: Transcatheter double valve replacement can be performed through a transapical access. The key to success is thorough preoperative planning based on CT, not only for sizing, but also for estimating the anatomical relationship of the prostheses. However, late migration can be expected and may lead to LVOT obstruction.
Introduction
Transcatheter aortic valve replacement (TAVR) has become the treatment of choice in high-risk and inoperable patients and has shown superiority to conventional surgery in the PARTNER trial1 and many subsequent studies. Devices for interventional mitral valve replacement are in their preclinical or early clinical phases. However, transcatheter aortic prostheses have been used for implantation into degenerated mitral bioprostheses, annuloplasty rings and, less frequently, into native calcified valves2,3. So far, implantation into native mitral valves has not been recommended due to unpredictable and non-reproducible results.
Case report
Here we report on the first transcatheter double native valve implantation (aortic and mitral) using a single transapical access.
A 67-year-old highly symptomatic female patient was admitted with aortic valve stenosis (opening area 1.0 cm2, peak gradient 38 mmHg) and combined mitral valve disease with predominant stenosis (opening area 1.1 cm2, mean gradient 15 mmHg, mitral regurgitation was considered mild to moderate). Left ventricular (LV) function was normal (ejection fraction [EF] 60%). In addition, she had experienced several episodes of life-threatening bleeding due to a malignant bladder tumour during anticoagulation for atrial fibrillation. Surgery for the tumour had been refused because of her worsening cardiac condition.
The extreme operative risk resulted from chronic obstructive pulmonary disease (COPD) with pulmonary hypertension (systolic pressure 64 mmHg), renal insufficiency, dependency on a walking frame, and malignant disease. Severe calcification of the posterior mitral annulus involving part of the posterior LV wall was judged technically demanding, thus increasing operative time and risk. Combined TAVR and mitral valvuloplasty was not considered due to a high Wilkins score of 15 and the probability of fatally increasing mitral regurgitation. The decision to perform a transcatheter procedure was made by an interdisciplinary Heart Team including cardiac surgeons, cardiologists, cardiac anaesthesiologists and radiologists. The Heart Team considered it justified to take the risk of this “compassionate use” procedure because the patient was relatively young and, even with the comorbidities described, survival time from the oncological standpoint was considered to be far beyond one year.
Valve dimensions were calculated based on computed tomography (CT) using 3-mensio Structural Heart (3mensio Medical Imaging BV, Bilthoven, The Netherlands) and OsiriX software (Pixmeo SARL, Bernex, Switzerland). The mitral annulus (Figure 1A) appeared to be suitable for a 29 mm Edwards SAPIEN 3™ (Edwards Lifesciences, Irvine, CA, USA) and the aortic annulus for a 23 mm Edwards SAPIEN 3 (Edwards Lifesciences) valve. The anatomical relation of both valve annuli was visualised by multiplanar reconstruction from CT (Figure 1B, MPR double oblique). Figure 1C gives the dimensions of the aortic annulus.
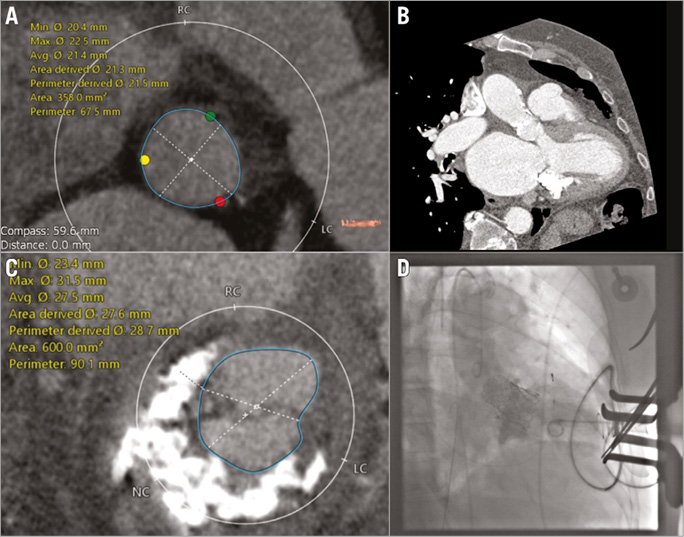
Figure 1. Preoperative diagnosis and first angiogram. A) Mitral annulus as reconstructed by double oblique multiplanar reconstruction (MPR) from CT. B) Anatomical relation of both valve annuli (MPR). C) Annular dimensions, aortic annulus. D) Left ventricular angiography to demonstrate competence of the mitral prosthesis.
The operation was performed under general anaesthesia in a hybrid operating room (OR) and was guided by fluoroscopy and 3D transoesophageal echocardiography (TEE). The apex was exposed through a typical left mini-thoracotomy, an APICA ASC™ closure system (St. Jude Medical, St. Paul, MN, USA) was inserted, and an Ascendra sheath (Edwards Lifesciences) suitable for the 29 mm device placed in the left ventricle. After antegrade crossing of the aortic valve and placement of a 0.035” guidewire in the descending aorta, a pre-shaped extra-stiff wire (Amplatz Super Stiff™; Boston Scientific Corp., Marlborough, MA, USA) was secured in the left atrium. The 29 mm prosthesis was expanded during rapid pacing, angiography and TEE both revealed no regurgitation into the left atrium, and the gradient was 4 mmHg (Figure 1D, LV angio).
Subsequently, the aortic prosthesis was implanted without prior balloon valvuloplasty in a typical transapical procedure. As in the mitral position, there was no regurgitation (Figure 2A, aortic angio) and a peak transvalvular gradient of less than 10 mmHg (both gradients as calculated from echocardiography; the competence of the prostheses was determined by echo and angiography). The combined LVOT/transaortic gradient was measured at less than 20 mmHg.
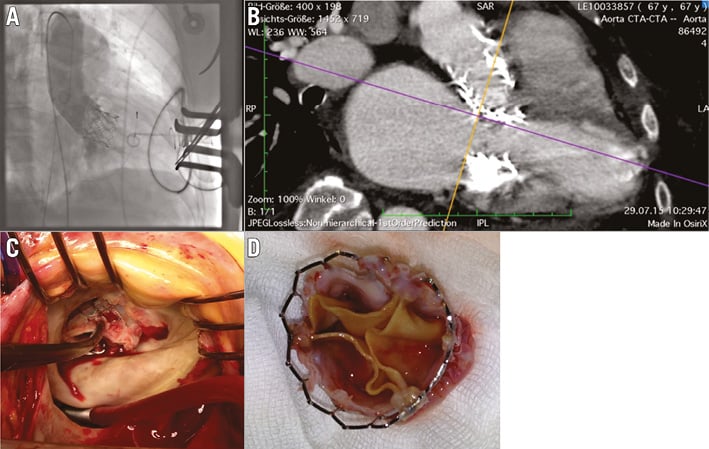
Figure 2. Second angiogram and postoperative situation. A) Aortic angiography to examine competence of the aortic prosthesis. B) Migration of the mitral prosthesis after five months (MSCT). C) Explantation of mitral prosthesis, operative view. D) Explanted mitral prosthesis.
The apex was closed with the APICA ASC system without complications. The patient was extubated a few hours after the intervention and transferred to the normal ward the following morning. Postoperative mobilisation and recovery was uneventful.
After five months, the patient again exhibited symptoms of cardiac failure, the reason being migration of the mitral prosthesis towards the LVOT (Figure 2B, MSCT). TEE revealed a gradient of 63 mmHg across the LVOT, paravalvular regurgitation being only trace. The mitral prosthesis showed a mean gradient of 4 mmHg and mild regurgitation. As the overall condition of the patient had meanwhile markedly improved, replacement of the mitral prosthesis by open heart surgery was possible (Figure 2C). The explanted prosthesis appeared to be slightly impressed at the region touching the aortic prosthesis, while the leaflets did not reveal any signs of degeneration (Figure 2D). Again, the patient recovered well, but unexpectedly died nine months after the initial valve replacement due to her malignant disease.
Discussion
For patients with left-sided double valve disease, open heart surgery is still the treatment of choice. In this patient with double valve disease, however, high operative risk had to be expected due to comorbidities and severe calcification of the posterior mitral annulus. In addition, fast recovery was desirable to enable surgery for her malignant tumour. To the best of our knowledge, this is the first successful attempt to perform transcatheter double valve implantation into native valves by a single transapical access. The balloon-expandable Edwards SAPIEN 3 prosthesis had been chosen due to its low profile and the downstream open stent structure, thus resulting in a low probability of obstructing the LVOT by the mitral prosthesis. However, one major disadvantage of this device is that, once deployed, retrieval of the prosthesis is not possible and open surgery would have been required. For that reason, thorough preoperative evaluation in close co-operation with the radiology department is mandatory.
In a theoretical discussion recently published in EuroIntervention4, implantation of the aortic before the mitral prosthesis was recommended due to the likelihood of obstructing the path to the aortic valve with the mitral device already in place. However, because of the anatomical proximity of both valves and the balloon-expandable design, a slight compression of the first prosthesis caused by the second might be expected. We chose to compress the larger mitral valve rather than the smaller aortic, and decided to implant the mitral first. However, respecting the considerations of Himbert et al, we placed a guidewire through the aortic valve from the beginning to facilitate access.
The case described reveals the feasibility and safety of double valve replacement into native valves from a single transapical access in selected patients at high or extreme risk for conventional surgery. Obviously, complete circular calcification of the mitral annulus is not necessary to ensure secure fixation of the mitral device. The key to success is thorough preoperative analysis based on CT, not only for sizing, but also to allow estimation of the interference of both prostheses.
However, even after a satisfying initial result, late migration of the mitral prosthesis is possible, so all efforts are justified to design more suitable prostheses for interventional mitral valve prostheses. The reason for the late embolisation of the mitral prosthesis is difficult to speculate on. Cardiopulmonary resuscitation (CPR) was never necessary in this patient. “Loosening’” of the mitral prosthesis by implantation of the aortic prosthesis seems unlikely, because the mitral prosthesis stayed in place for several months. There is indeed one report in the literature describing late migrations of SAPIEN valves in the mitral position; however, the cases published by Bapat and co-workers described valve-in-valve interventions5. Migration of a SAPIEN prosthesis implanted into a native annulus has not been described so far.
| Impact on daily practice Top-down implantation of aortic prostheses has frequently been performed in the mitral position. However, as in many previous cases, and also in the case described here, it can only be considered a temporary solution. For mitral valve replacement, every effort to develop adequate prostheses is justified. |
Conflict of interest statement
R. Bauernschmitt is a proctor/consultant for Medtronic, JenaValve, Direct Flow Medical and Edwards. The other authors have no conflicts of interest to declare.

