
Abstract
Aims: Concomitant severe calcific aortic and mitral stenosis is a relatively uncommon but very challenging valvular heart disease to manage. We sought to evaluate the feasibility of a fully percutaneous approach to replace both stenotic native mitral and aortic valves using SAPIEN 3 valves.
Methods and results: An 87-year-old woman with chronic kidney disease stage 3, pulmonary hypertension, chronic obstructive pulmonary disease, a permanent pacemaker, and atrial fibrillation was referred with Class III heart failure symptoms. Her echocardiogram showed a decreased ejection fraction at 45%, severe mitral stenosis (mean gradient 13 mmHg, area 0.8 cm2) with severe MAC, and severe AS (mean gradient 35 mmHg, area 0.6 cm2). Surgical risk was felt to be very high after evaluation by our cardiothoracic surgery group (Society of Thoracic Surgeons risk score of 19%). She underwent simultaneous and fully percutaneous transfemoral TAVR and transseptal TMVR using SAPIEN 3 valves. Post-implant TEE showed trace paravalvular mitral regurgitation and a mean gradient of 4 mmHg and mean aortic gradient of 8 mmHg with trace paravalvular leak. There was no LVOT obstruction. The patient was discharged seven days after the intervention.
Conclusions: After careful evaluation by experienced Heart Teams, combined native stenotic mitral and aortic valves can be percutaneously replaced using transcatheter SAPIEN 3 valves via transfemoral access in carefully selected high surgical risk patients.
Introduction
Transcatheter mitral valve replacement using SAPIEN 3 valves (Edwards Lifesciences, Irvine, CA, USA) has been performed in degenerative bioprosthetic mitral valves, mitral rings, and occasionally in native mitral valves with heavy calcification1. Concomitant severe aortic and mitral stenosis (MS) in patients who are not candidates for traditional surgery or percutaneous balloon mitral valvuloplasty is a complex scenario that we will encounter more commonly with an aging population. Although simultaneous transapical transcatheter double valve replacement has been reported before2, simultaneous transcatheter native double valve replacement via the transfemoral access has not been described.
Methods
An 87-year-old woman with chronic kidney disease stage 3, pulmonary hypertension, chronic obstructive pulmonary disease, a permanent pacemaker, and atrial fibrillation was referred to our institution with Class III heart failure symptoms. Her echocardiogram showed a decreased ejection fraction at 45%, severe mitral stenosis (mean gradient 13 mmHg, mitral valve area of 0.8 cm2) with severe mitral annular calcification (MAC), and severe aortic stenosis (AS) (mean gradient 35 mmHg, aortic valve area 0.6 cm2).
A coronary angiogram showed a chronically occluded non-dominant right coronary artery and non-obstructive disease in the left coronary system.
Computed tomography demonstrated a nearly circumferential distribution of MAC (Figure 1). The surgical risk was felt to be very high after evaluation by our cardiothoracic surgery group (Society of Thoracic Surgeons risk score of 19%). Our multidisciplinary valve team recommended simultaneous transcatheter mitral valve replacement (TMVR) through a transseptal (TS) approach and transcatheter aortic valve replacement (TAVR) through a transfemoral approach.
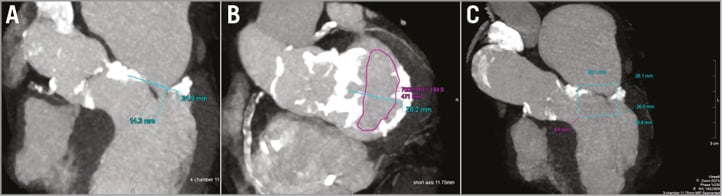
Figure 1. Cardiac computed tomography (CT) images showing a heavily calcified mitral and aortic annulus (A) with a mitral annulus area of 471 mm2 suitable for a 26 mm SAPIEN 3 valve (B) without LVOT obstruction using virtual testing of a computer-generated SAPIEN 3 valve (C).
First, the TAVR procedure was performed percutaneously from the left femoral artery using a 23 mm SAPIEN 3 valve (Edwards Lifesciences) under general anaesthesia and with transoesophageal echocardiography (TEE) and fluoroscopy guidance. The arteriotomy site was pre-closed using two Perclose devices (Abbott Vascular, Santa Clara, CA, USA). Then, the TMVR procedure was performed from the right femoral vein. After transseptal puncture and dilatation of the atrial septum with a 20 mm balloon (Armada; Abbott Vascular), a shaped Amplatz Super-Stiff guidewire (Cook Medical, Bloomington, IN, USA) was positioned in the left ventricle. A 26 mm SAPIEN 3 valve was advanced to the annulus, positioned with fluoroscopic and TEE guidance, and then successfully implanted within the mitral annulus by slowly inflating the balloon during rapid pacing (Figure 2, Moving image 1-Moving image 5). At the end of the procedure the Perclose sutures were tightened and venous puncture was closed using a purse-string suture.
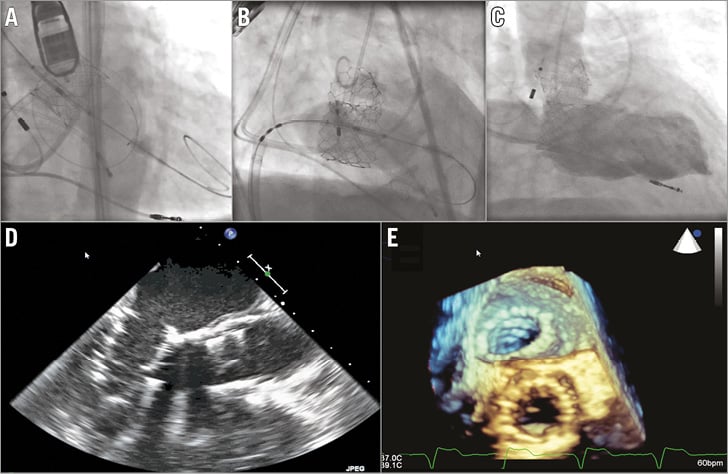
Figure 2. Transfemoral aortic valve replacement and transseptal mitral valve replacement. A) Implantation of a 23 mm SAPIEN 3 valve in the aortic position (Moving image 1). B) Implantation of a 26 mm SAPIEN 3 valve in the mitral position (Moving image 2). C) Ventriculogram showing mild paravalvular mitral regurgitation (Moving image 3). D) Transoesophageal echocardiogram showing functioning SAPIEN 3 valves in the mitral and aortic positions (Moving image 4) with no significant paravalvular leak on colour Doppler (Moving image 5). E) Post-implantation 3-dimensional transoesophageal echocardiogram.
Post-implant TEE showed trace paravalvular mitral regurgitation and a mean gradient of 4 mmHg and mean aortic gradient of 8 mmHg with trace paravalvular regurgitation. There was no left ventricular outflow (LVOT) obstruction. The postoperative course was complicated by a transient ischaemic attack, and the patient was discharged seven days after the intervention.
At one-month follow-up she had only Class II heart failure symptoms. Repeat transthoracic echocardiography at that time showed a mean mitral valve gradient of 6 mmHg, and mean aortic valve gradient of 8 mmHg with trace paravalvular regurgitation across both valves; the LVOT gradient was 4 mmHg and right ventricular systolic pressure was estimated at 47 mmHg. The patient passed away three months after the procedure.
Discussion
TAVR has emerged as a new lifeline for patients with severe aortic stenosis who are inoperable or high risk for surgical aortic valve replacement. However, little can be offered to inoperable patients with severe MS who are not candidates for surgical mitral valve replacement. Some centres have reported experience with concurrent transapical mitral and aortic valve implantation in patients who have failed prosthetic mitral and aortic valves. There is also a recent report of successful implantation of transapical mitral and aortic valve replacements in native valves3-6.
To our knowledge, we report here the first case of successful treatment by a simultaneous and fully percutaneous transfemoral approach for a patient with severely stenotic native mitral and aortic valves using SAPIEN 3 valves.
The challenges of TMVR are sizing the annulus, the amount of calcification required to anchor the prosthesis, and predicting LVOT obstruction caused by the anterior mitral valve leaflet. Although there is no consensus about how to measure the mitral annulus, in our case the mitral annulus was heavily calcified; CT and TEE predicted annulus measurements that were satisfactory for a 26 mm SAPIEN 3 valve based on the aortic sizing chart for the SAPIEN 3. Once the valve size had been identified, a SAPIEN 3 valve generated by computer-aided design was then tested virtually to confirm the sizing and to estimate the risk of LVOT obstruction.
Transseptal access can complicate manoeuvring the valve perpendicular to the mitral annulus plane. In this case, we used a balloon to dilate the atrial septum and facilitate valve crossing. We also used the flexibility of the Edwards Commander Delivery System (Edwards Lifesciences) to adjust the valve position and perpendicularity within the mitral annulus. After the procedure, there was a small residual shunt detected by TEE across the interatrial septum, but it was not evident on the one-month transthoracic echocardiogram. The use of a fully percutaneous transfemoral treatment allows shortening of the hospital stay, thus avoiding complications related to thoracotomy or transapical access.
Conclusions
After careful evaluation by experienced Heart Teams, combined native valve TAVR/TMVR can be performed via the transfemoral access in carefully selected patients. This therapeutic option may be considered as a valuable alternative to conventional surgery in extremely high-risk patients with concomitant mitral and aortic valve stenosis.
| Impact on daily practice To this day most patients with concomitant aortic and mitral valve disease are treated surgically. With the advances in technology, transcatheter double valve replacement is emerging as an alternative for highly selected individuals who are high risk for surgical therapy. The key to success is a multidisciplinary approach with focus on valve sizing, amount of mitral calcification and the appropriate method of delivery. LVOT obstruction remains a big challenge and requires careful procedural planning and patient selection. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Implantation of a 23 mm SAPIEN 3 valve in the aortic position.
Moving image 2. Implantation of a 26 mm SAPIEN 3 valve in the mitral position.
Moving image 3. Ventriculogram showing mild paravalvular mitral regurgitation.
Moving image 4. Transoesophageal echocardiogram showing functioning SAPIEN 3 valves in the mitral and aortic positions.
Moving image 5. Transoesophageal echocardiogram showing functioning SAPIEN 3 valves in the mitral and aortic positions with no significant paravalvular leak on colour Doppler.
Supplementary data
To read the full content of this article, please download the PDF.
Implantation of a 23 mm SAPIEN 3 valve in the aortic position.
Implantation of a 26 mm SAPIEN 3 valve in the mitral position.
Ventriculogram showing mild paravalvular mitral regurgitation.
Transoesophageal echocardiogram showing functioning SAPIEN 3 valves in the mitral and aortic positions.
Transoesophageal echocardiogram showing functioning SAPIEN 3 valves in the mitral and aortic positions with no significant paravalvular leak on colour Doppler.

