Abstract
In high-risk patients with aortic stenosis and associated cardiac comorbidities (such as coronary artery disease, atrial fibrillation or combined valve disease), transcatheter interventions offer a unique opportunity to mitigate these individuals’ cardiovascular risk, either by staging the interventions, or by performing simultaneous procedures in a single session. The decision on which approach (staged vs. single session) to choose for an individual patient depends on clinical, anatomical and patient-related factors. While a staged approach may represent a preferable strategy in selected patients, concomitant treatment of combined cardiac diseases represents an appealing option in a majority of patients.
Case vignette
A 91-year-old lady in permanent atrial fibrillation and acute heart failure class IV was found to suffer from severe mitral regurgitation (MR) and structural degeneration of a surgical aortic bioprosthesis implanted 11 years earlier (Carpentier-Edwards 21 mm; Edwards Lifesciences, Irvine, CA, USA). At that time, she also received two aortocoronary venous grafts. During the last six months she had two hospitalisations for heart failure, but was previously very active and living independently.
Transoesophageal echocardiography (TEE) showed severe degenerative mitral regurgitation (MR) due to a posterior flail leaflet with severe pulmonary hypertension (60 mmHg). Left ventricular ejection fraction (EF) was 50%. The aortic bioprosthesis was severely calcified with extremely reduced mobility (prosthetic mean gradient 25 mmHg, peak 40 mmHg) (Figure 1). Stress echo was not feasible because of the severe MR and pulmonary hypertension but, based on the valve morphology (severe calcification, leaflet immobility) and considering that EF was at the lower limit in the presence of severe MR, the aortic stenosis (AS) was judged as severe (low-flow low-gradient).
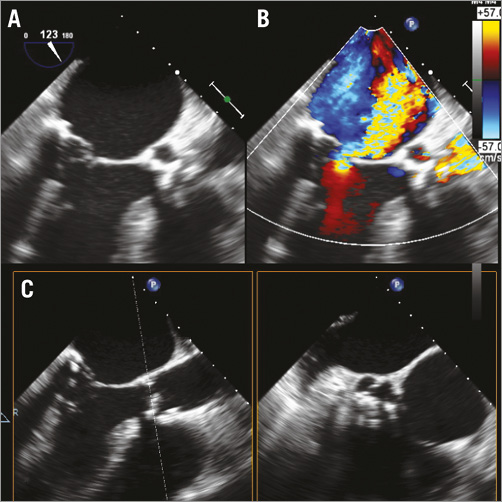
Figure 1. Pre-procedural TEE. A) & B) Transoesophageal echocardiography (LVOT view) with colour Doppler showing severe eccentric mitral regurgitation due to a flail posterior leaflet. C) X-plane of the degenerate aortic bioprosthesis showing minimal opening area during systole.
The patient was taking long-term oral anticoagulation because of her permanent atrial fibrillation (AF) (CHA2DS2-VASc score 5) but was also at high risk of bleeding (HAS-BLED score 4).
After discussing the possible options with the patient and her family, she was scheduled to undergo transcatheter aortic valve implantation (TAVI), MitraClip implantation and left atrial appendage occlusion (LAAO) in a single session.
As a first step, the aortic valve-in-valve procedure was successfully performed using a CoreValve® 23 mm (Medtronic, Minneapolis, MN, USA) bioprosthesis. Mean transaortic gradient was 10 mmHg, and there was only a trivial paravalvular leak. This was followed by transseptal puncture and implantation of two MitraClip® (Abbott, Abbott Park, IL, USA) devices, resulting in only mild residual MR and no significant mitral gradient. Thirdly, the steerable guide catheter of the MitraClip system was replaced by a 13 Fr 45×45 TorqVue™ sheath (St. Jude Medical, St. Paul, MN, USA) for LAAO using a 26 mm AMPLATZER™ Cardiac Plug (St. Jude Medical) (Figure 2).
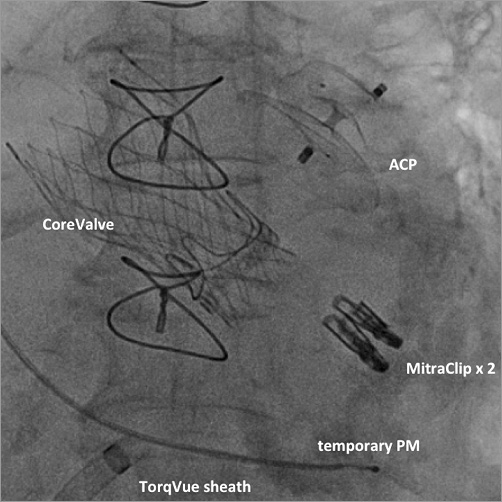
Figure 2. Final angiogram showing the CoreValve 23 mm valve, two MitraClip devices and the AMPLATZER Cardiac Plug (ACS) in place.
The post-procedural course was smooth with discharge on day seven. Pre-discharge echocardiography showed a significant reduction in pulmonary pressure (45 mmHg) and an EF of 40%. At one-year follow-up, the patient is asymptomatic (NYHA I) and living independently, free of anticoagulants and any major adverse event.
Introduction
Transcatheter aortic valve implantation (TAVI) is a valid alternative to open heart surgery in different clinical settings, mainly in patients deemed high risk for conventional aortic valve replacement1,2. This is particularly true among patients with coexisting cardiac diseases, which would require complex combined surgical management and additional operative risk. In patients with associated cardiac comorbidities (such as coronary artery disease, atrial fibrillation or combined valve disease), transcatheter interventions offer a unique opportunity to mitigate these individuals’ overall cardiovascular risk, either by staging the interventions, or by performing simultaneous procedures in a single session. While a staged approach may represent a preferable strategy in selected patients, the technical feasibility of concomitant treatment of combined cardiac diseases in a single session has been demonstrated. This approach may represent an appealing and patient-friendly option in many cases3. The decision as to which approach (staged versus single session) to choose for an individual patient depends on clinical, anatomical and patient-related factors:
– Which is the most clinically relevant problem?
– Are all the concomitant conditions clinically relevant or is a “skilful omission” strategy preferable?
– Can one condition improve after treatment of the most relevant clinical problem (e.g., improvement of functional mitral regurgitation after TAVI)?
– Are all the procedures technically feasible?
– In which order should the concomitant procedures be performed?
TAVI and MitraClip
MR is frequently found in patients with AS scheduled for TAVI. The aetiology of concomitant MR can be organic or, more frequently, functional. Surgical experience has shown reduced survival in patients with at least moderate MR undergoing surgical aortic valve replacement (AVR)4,5. Interestingly, two-year results from the PARTNER I trial (Cohort A) showed that concomitant moderate to severe baseline MR was associated with increased mortality in the surgical group only, while this association was not observed in patients undergoing TAVI6. However, no information about the aetiology of concomitant MR was reported.
There is general agreement that concomitant MR should be treated when it is severe, especially when of degenerative aetiology. While functional non-severe MR is likely to decrease after correction of AS, degenerative MR usually remains unchanged.
As shown in the case vignette and in our local experience, double transcatheter valve treatment of patients with coexisting AS and MR is feasible and safe in a single session. The decision between a staged versus single-step approach should be tailored to the individual patient, taking into consideration age, comorbidities, severity of MR and its aetiology, the likelihood of spontaneous improvement, feasibility of MitraClip implantation and the risk of future interventions. In patients in whom severe MR dominates the clinical scenario and the likelihood of improvement in MR after TAVI is minimal (as in the case vignette), our strategy is to perform the procedures concomitantly. TAVI is performed as the first step, followed by MitraClip. To minimise the time with a large device in the iliofemoral arteries, we typically remove the arterial sheath before starting the MitraClip procedure.
TAVI and LAA occlusion
Concomitant AF is independently associated with late cardiovascular morbidity and mortality after TAVI7-9, mainly as a result of the increased risks of stroke and of bleeding related to oral anticoagulant therapy. Bleeding risk is particularly high in patients with older age, hypertension, previous stroke and diabetes, all of which have high prevalence in the TAVI population10. One-year mortality of patients in AF in the PARTNER I trial was double (26.2%) that of patients in sinus rhythm (12.9%) and quadrupled (48.7%) if these patients experienced a major bleeding complication within the first year of follow-up11. These worrying data indicate that almost every second TAVI patient in AF who experiences a major bleeding complication will be dead after one year.
Transcatheter LAAO not only provides better stroke prevention than warfarin, but a significant 34% mortality reduction12. Therefore, concomitant LAAO is an attractive solution for patients undergoing TAVI who are in AF and at high risk of bleeding. Ad hoc LAAO can be performed using fluoroscopic guidance alone, omitting general anaesthesia and TEE13. Although the combined procedure requires a slightly higher volume of contrast medium, this does not appear to impact on periprocedural morbidity and mortality3. The major advantages of performing TAVI and LAAO as concomitant procedures are the immediate protection from stroke (maximum risk of stroke is in the first 24 hours after TAVI) and bleeding complications1,7. Furthermore, discontinuation of oral anticoagulants is particularly appealing in patients in atrial fibrillation who also need dual antiplatelet therapy for coexistent coronary artery disease (CAD), particularly in the setting of a recent percutaneous coronary intervention (PCI).
TAVI and PTCA
More than 50% of patients with AS aged >70 years have associated CAD14, and its prevalence increases with age and in the presence of aortic valve calcification15. The prevalence of associated CAD in patients undergoing TAVI is therefore particularly high. However, the optimal management of CAD in patients undergoing TAVI remains uncertain. Staged or simultaneous PCI and TAVI have been shown to be safe and feasible in different series16,17.
PCI before TAVI has the potential to reduce the procedural risk of TAVI as well as the need for subsequent revascularisation. Moreover, clinical outcomes of patients treated using a staged approach (PCI before TAVI) do not differ from those of patients treated concomitantly16.
Potential advantages of the concomitant approach include enhanced resource utilisation, patient convenience, and increased safety due to use of the same arterial access site for both TAVI and PCI. Investigators have also explored the possibility of performing PCI immediately after TAVI18, but this strategy raises several concerns, including the possibility that the prosthetic valve struts may interfere with guide catheter engagement of the coronary arteries.
TAVI and other structural procedures
Patent foramen ovale (PFO) closure has been shown to be more effective than medical therapy for secondary stroke prevention19,20. However, PFO closure for primary stroke prevention is controversial and data are limited. If a PFO is present, it should be used as a means of left atrial access when concomitant LAAO is performed21 and can be closed on the way out. A residual atrial septal defect following transseptal puncture does not require intervention, except when associated with bidirectional shunting, desaturation and heart failure.
Conclusions
Transcatheter treatment of combined cardiac problems at the time of TAVI is feasible in a single session. Furthermore, this approach seems to be safe when undertaken by experienced operators in high-volume centres. A single-session (rather than a staged) approach has several advantages for the patient: this is an important consideration for elderly, often multimorbid individuals (and their families). Moreover, selected combined procedures (excluding MitraClip) may be performed without general anaesthesia and TEE guidance.
There is a need for a larger randomised trial of combined versus staged procedures to compare these approaches.
Last but not least, whilst a combined approach may be patient-friendly, it is financially disadvantageous for the treating institution. Most national and local healthcare funding systems do not recognise or reimburse combined procedures and this may prevent (or delay) the adoption of this strategy in many centres. Hopefully, this illogical position will be addressed and corrected in the near future.
Conflict of interest statement
F. Maisano is a consultant for Abbott Vascular, Medtronic, St Jude, Valtech Cardio, receives royalties from Edwards Lifesciences and is co-founder of 4Tech. F. Nietlispach is a consultant for Edwards and Biotronix. M. Taramasso has no conflicts of interest to declare.

