An 80-year-old male patient with symptomatic, severe aortic stenosis, coronary artery disease and permanent atrial fibrillation was referred for transcatheter aortic valve implantation (TAVI). After staged percutaneous coronary intervention of the LAD with drug-eluting stents, he suffered from recurrent lower gastro-intestinal bleeding under triple anticoagulation (acetylsalicylic acid, clopidogrel, and phenprocoumon). Due to high surgical risk (STS score 11.8%) and high risk for stroke (CHA2DS2-VASC score 6) and recurrent bleeding (HASBLED score 4), he underwent transfemoral TAVI (CoreValve 29 mm bioprosthesis; Medtronic, Minneapolis, MN, USA) and closure of the left atrial appendage (Watchman 27 mm device; Boston Scientific, Natick, MA, USA) with periprocedural use of a novel cerebral protection device (CE Pro; Claret Medical Inc., Santa Rosa, CA, USA) via right-sided transbrachial access. The proximal and distal filters were deployed within the innominate artery and the left common carotid artery, respectively (Figure 1A and Figure 1B). TAVI and LAA closure under TEE guidance were then successfully performed (Figure 1C - Figure 1E). After retrieval of the cerebral protection device, debris and embolic material were found to be captured within the filters (Figure 1F). The patient was discharged uneventfully on day six.
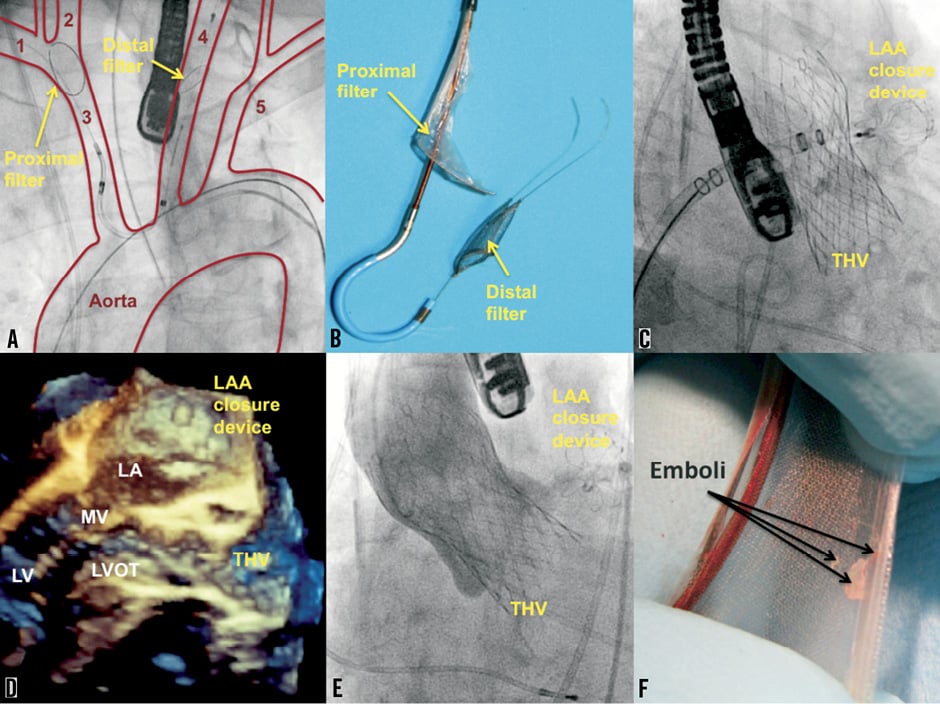
Figure 1. TAVI and closure of the left atrial appendage under cerebral protection. A) 1. right subclavian artery; 2. right carotid artery; 3. brachiocephalic trunk (innominate artery) ; 4. left carotid artery; 5. left subclavian artery. B) Claret CE Pro cerebral protection device with proximal and distal filer. C) LAA closure with Watchman device after TAVI. D) Three-dimensional echocardiographic control after successful TAVI and LAA closure. E) Angiography of the aorta with only trivial peri-prosthetic aortic regurgitation after TAVI. F) Debris in the proximal filter after the procedure. LA: left atrium; LAA: left atrial appendage; LV: left ventricle; LVOT: left ventricular outflow tract; MV: mitral valve; THV: transcatheter heart valve
Not only transcatheter heart valve interventions but also other structural heart procedures with transseptal puncture and introduction of a sheath into the left atrium for closure of the LAA or mitral valve interventions bear the potential risk of periprocedural cerebral embolism and stroke that may be reduced by use of a dedicated cerebral protection device.
Conflict of interest statement
E. Grube is a proctor for CoreValve/Medtronic. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Cerebral protection device in place.

