
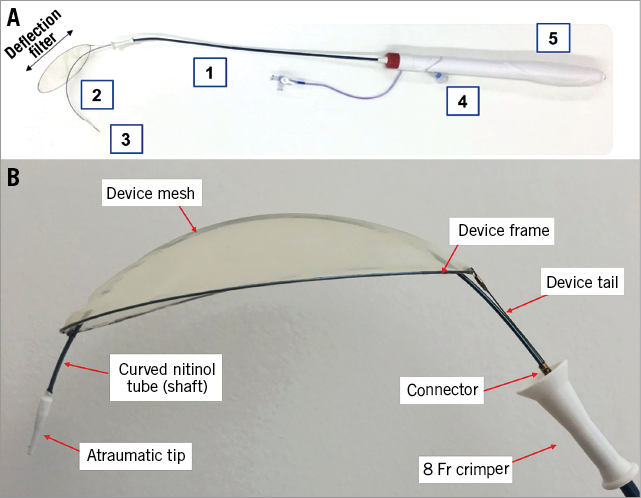
Figure 1. TriGUARD 3 cerebral embolic protection device. A) The TriGUARD 3 system: 1) 8 Fr integrated delivery sheath; 2) nitinol shaft to provide stability after deployment; 3) atraumatic tip to accommodate the guidewire for an over-the-wire delivery; 4) integrated pigtail entry port; 5) ergonomic handle to allow consistent deployment and retrieval. B) TriGUARD 3 deflection filter.
Clinical neurological events after TAVI occur in ~4% of patients1. Cerebral embolic protection devices (CEPD) have recently been developed to reduce the risk of stroke during transcatheter aortic valve implantation (TAVI)2-4. The new TriGUARD 3™ system (Keystone Heart, Caesarea, Israel) (Figure 1) is a single-use, temporary, biocompatible filter made of polyetheretherketone (PEEK) mesh and a nitinol frame, which is delivered via a smaller, 8 Fr integrated sheath in the femoral artery. The upper and lower stabilisers have been removed and the nitinol frame now has a self-positioning and self-stabilising capability. The filter area has increased from 20.9 cm2 to 68.3 cm2 in the new-generation device. The tip of the system is atraumatic and now accommodates a guidewire for an over-the-wire (OTW) delivery. The new-generation device has an integrated pigtail entry port and a handle to allow more consistent deployment and retrieval of the device. The self-positioning filter portion of the device covers all three major cerebral arteries in the aortic arch, maintaining blood flow to the cerebral vessels through 115 µm pores. The filter is coated with an antithrombotic coating (Surmodics, Eden Prairie, MN, USA). We describe here the first-in-human case with the new-generation TriGUARD 3 CEPD.
An 87-year-old man was admitted to our institution with severe dyspnoea. His past medical history was significant for stage IV chronic kidney disease, hypertension, and ischaemic cardiomyopathy (left ventricular ejection fraction of 35%). A transthoracic echocardiogram revealed a severely stenotic aortic valve. A multislice computed tomography (MSCT) scan revealed a very high aortic valve calcium score (4,833 Agatston units) and an aortic annulus area of 469 mm2. The Heart Team elected to proceed with transfemoral TAVI with a 26 mm SAPIEN 3 transcatheter heart valve (THV) (Edwards Lifesciences, Irvine, CA, USA) with temporary cerebral embolic protection with the TriGUARD 3 CEPD. To make sure that the patient was a candidate for a TriGUARD 3, the MSCT scan had previously been analysed to exclude severe peripheral arterial, abdominal or thoracic aortic disease, as well as a severely tortuous or atheromatous aortic arch.
The procedure was performed under general anaesthesia (Moving image 1-Moving image 18). After deployment of the temporary CEPD and implantation of the THV, the patient was extubated in the procedure room with no neurological deficits or vascular complications. He was discharged home on day six.
The design features of the new-generation TriGUARD 3 device are expected to improve generalisability and ease of use, and enhance embolic protection properties. Future trials will determine the efficacy of this device for preventing cerebral embolism during TAVI procedures.
Conflict of interest statement
J. Rodés-Cabau holds the Canadian Research Chair “Fondation Famille Jacques Larivière” for the Development of Structural Heart Disease Interventions and has received institutional research grants from Edwards Lifesciences, Medtronic and Heart Leaflet Technologies. T. Nazif is an investigator for the TriGUARD 3 US trial. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Angiogram showing severe tortuosity of the iliofemoral vessels
Moving image 10. Co-planar view aligning the nadir of the three aortic cusps on the same plane.
Moving image 11. Subsequently, the 14 Fr E-sheath was advanced over the Meier wire in the right femoral artery, and an AL1 catheter was advanced to the ascending aorta with care to ensure that it remained beneath the TriGUARD 3 CEPD.
Moving image 12. A 26 mm SAPIEN 3 THV and Commander delivery system (Edwards Lifesciences) were then inserted through the E-sheath and over the Confida™ wire (Medtronic, Minneapolis, MN, USA) coming from the right femoral artery. The TriGUARD 3 system remained very stable in the intended position as the SAPIEN 3 valve and delivery system were advanced across the aortic arch and positioned in the aortic annulus.
Moving image 13. A 26 mm SAPIEN 3 THV and Commander delivery system (Edwards Lifesciences) were then inserted through the E-sheath and over the Confida™ wire (Medtronic, Minneapolis, MN, USA) coming from the right femoral artery. The TriGUARD 3 system remained very stable in the intended position as the SAPIEN 3 valve and delivery system were advanced across the aortic arch and positioned in the aortic annulus.
Moving image 14. Deployment of the 26 mm SAPIEN 3 transcatheter heart valve.
Moving image 15. Aortogram post TAVI showing mild paravalvular aortic regurgitation.
Moving image 16. Retrieval of the Edwards Commander delivery system without disturbing the TriGUARD 3 CEPD.
Moving image 17. Removal of the pigtail catheter.
Moving image 18. Re-sheathing process with use of the device handle and retrieval of the TriGUARD 3 CEPD.
Moving image 2. Aortogram performed to determine the exact location of the three main cerebral vessels.
Moving image 3. The TriGUARD 3 device and integrated 8 Fr sheath were advanced over the wire placed through the left femoral access.
Moving image 4. Advancing the TriGUARD 3 system in the abdominal and descending thoracic aorta.
Moving image 5. Advancing the TriGUARD 3 system across the aortic arch.
Moving image 6. The leading edge of the TriGUARD 3 filter element was positioned approximately 2-3 cm proximal to the innominate artery ostium, and the device was deployed using the handle to unsheath the filter.
Moving image 7. Deployment of the TriGUARD 3 (part 2).
Moving image 8. A pigtail catheter for procedural guidance was then inserted through the dedicated entry port of the CEPD system and advanced to the aortic root.
Moving image 9. Rotational fluoroscopy showing the well-positioned filter portion of the TriGUARD 3, the nitinol shaft, and the appropriate position of the pigtail catheter.

