Abstract
Aims: This study aimed to evaluate the safety and performance of the TriGuard™ Embolic Deflection Device (EDD), a nitinol mesh filter positioned in the aortic arch across all three major cerebral artery take-offs to deflect emboli away from the cerebral circulation, in patients undergoing transcatheter aortic valve replacement (TAVR).
Methods and results: The prospective, multicentre DEFLECT I study (NCT01448421) enrolled 37 consecutive subjects undergoing TAVR with the TriGuard EDD. Subjects underwent clinical and cognitive follow-up to 30 days; cerebral diffusion-weighted magnetic resonance imaging (DW-MRI) was performed pre-procedure and at 4±2 days post procedure. The device performed as intended with successful cerebral coverage in 80% (28/35) of cases. The primary safety endpoint (in-hospital EDD device- or EDD procedure-related cardiovascular mortality, major stroke disability, life-threatening bleeding, distal embolisation, major vascular complications, or need for acute cardiac surgery) occurred in 8.1% of subjects (VARC-defined two life-threatening bleeds and one vascular complication). The presence of new cerebral ischaemic lesions on post-procedure DW-MRI (n=28) was similar to historical controls (82% vs. 76%, p=NS). However, an exploratory analysis found that per-patient total lesion volume was 34% lower than reported historical data (0.2 vs. 0.3 cm3), and 89% lower in patients with complete (n=17) versus incomplete (n=10) cerebral vessel coverage (0.05 vs. 0.45 cm3, p=0.016).
Conclusions: Use of the first-generation TriGuard EDD during TAVR is safe, and device performance was successful in 80% of cases during the highest embolic-risk portions of the TAVR procedure. The potential of the TriGuard EDD to reduce total cerebral ischaemic burden merits further randomised investigation.
Abbreviations
ADC: apparent diffusion coefficient
DW-MRI: diffusion-weighted magnetic resonance imaging
EDD: embolic deflection device
GFR: glomerular filtration rate
HITS: high-intensity transient signals
MACCE: major adverse cardiovascular and cerebrovascular events
MoCA: Montreal Cognitive Assessment
mRS: modified Rankin scale
NS: not significant
OPC: objective performance criterion
TAVR: transcatheter aortic valve replacement
TCD: transcranial Doppler ultrasound
TIA: transient ischaemic attack
VARC: Valve Academic Research Consortium
Introduction
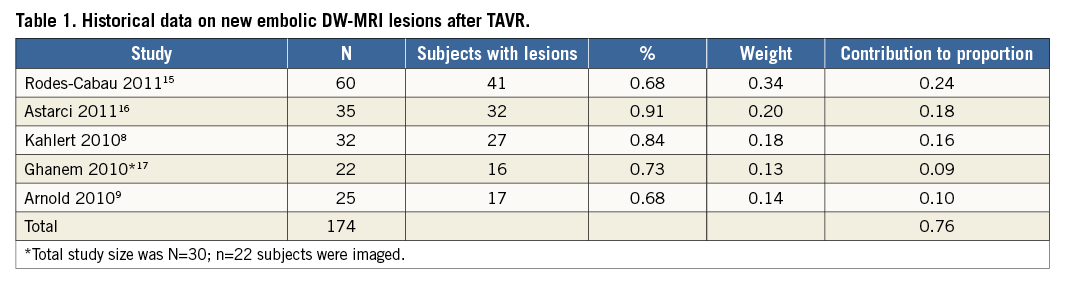
Stroke is a morbid complication of endovascular procedures and is a strong predictor of mortality1,2. The reported incidence of periprocedural stroke in transcatheter aortic valve replacement (TAVR) ranges from 1.5% to 10%3-5; however, clinically evident stroke may represent only one extreme of a spectrum of adverse neuro-embolic outcomes. Transcranial Doppler ultrasound (TCD) detects embolic signals in 100% of patients undergoing TAVR6,7, and 68-91% of patients have new post-procedure foci of restricted diffusion on diffusion-weighted magnetic resonance imaging (DW-MRI) (Table 1)8-10. The presence of these clinically silent brain infarcts has been linked to dementia, cognitive decline, and an increased risk of subsequent overt stroke11,12. Prevention of neurological complications would further improve outcomes of TAVR in patients with severe symptomatic aortic stenosis (AS).
Methods
STUDY DESIGN AND PATIENT POPULATION
DEFLECT I is a prospective, multicentre, single-arm study designed to evaluate the safety and performance of the TriGuard™ Embolic Deflection Device (EDD) (Keystone Heart Ltd., Herzliya, Israel) in patients undergoing TAVR at six investigational sites in the European Union and Brazil. Eligible subjects were adults presenting with indications for TAVR (severe symptomatic AS and high or extreme surgical risk). Key exclusion criteria included recent (<72 hours) acute myocardial infarction, recent (<6 months) stroke or transient ischaemic attack (TIA), cardiogenic shock, impaired renal function (glomerular filtration rate [GFR] <30 mg/dl), past or pending organ transplant, active peptic ulcer or recent (<6 months) gastrointestinal bleeding, and history of bleeding diathesis or coagulopathy or contraindications to antiplatelet or anticoagulant therapy. Patients were also excluded if they were undergoing TAVR via the transaxillary, subclavian, or direct aortic route, had known hypersensitivity to device component materials or contrast that could not be adequately premedicated, severe peripheral artery disease that precluded vascular access, contraindications to cerebral MRI, or planned treatment with any other investigational device or procedure. The trial was approved by the institutional review board or medical ethics committee at each site and all subjects provided written informed consent.
STUDY DEVICE
The TriGuard EDD is a temporary, single-use, biocompatible filter made of fine nitinol (nickel titanium alloy) wires, which is delivered transfemorally via a 9 Fr Mullins introducer sheath, positioned in the aortic arch, and anchored in position by an atraumatic stabiliser in the ostium of the innominate artery (Figure 1). The filter portion of the device covers all three major cerebral arteries in the aortic arch (innominate, left common carotid and subclavian), maintaining blood flow to the cerebral vessels through 250 µm pores while deflecting larger emboli to the descending aorta. The device is intended to reduce the passage of embolic material to the cerebral arteries during endovascular procedures. The filter is coated with an antithrombotic coating (Applause™ Heparin Coating; SurModics, Inc., Eden Prairie, MN, USA).
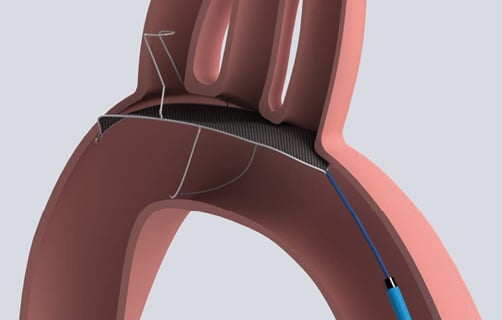
Figure 1. The Keystone Heart TriGuard Embolic Deflection Device. Illustration of device positioning in the aortic arch.
PROCEDURE
TAVR was performed with the SAPIEN transcatheter heart valve (Edwards Lifesciences, Irvine, CA, USA) or the CoreValve® transcatheter aortic valve (Medtronic, Minneapolis, MN, USA), according to standard institutional procedures via a transapical or transfemoral approach under local or general anaesthesia. Standard dual antiplatelet therapy with aspirin (300-325 mg loading dose and 75-325 mg daily maintenance dose indefinitely) and clopidogrel (≥300 mg loading dose >6 hours before the procedure or 600 mg periprocedurally, and 75 mg daily maintenance dose for ≥6 months) was employed.
At the start of the procedure, a 9 Fr arterial sheath was inserted in the contralateral femoral artery (either femoral artery if the transapical approach was employed), through which the EDD was advanced to the aortic arch and deployed to cover the ostia of the three major cerebral vessel take-offs. The EDD was withdrawn after completion of the TAVR procedure.
All subjects underwent clinical and cognitive screening at baseline, post procedure, and at 30 days. Neurological and cognitive assessments included standard clinical scales (the National Institutes of Health Stroke Scale [NIHSS] and the modified Rankin Scale [mRS]) as well as the Montreal Cognitive Assessment (MoCA)13. Staff who performed the neurological assessments were NIHSS certified, trained in administration of the mRS and MoCA, and blinded to DW-MRI findings. DW-MRI was performed at baseline and post procedure (4±2 days). Independent site monitoring was performed for 100% of clinical fields and clinical events.
ENDPOINTS
The primary device performance endpoint (adjudicated by an independent angiographic core laboratory: Yale Cardiovascular Research Group, New Haven, CT, USA) was defined as the ability to perform the following functions without device malfunction: 1) access the aortic arch with the delivery catheter, 2) deploy the EDD, 3) position the device to cover all three cerebral inflow vessels without obstruction of blood flow or interference with the TAVR procedure, and 4) retrieve the intact device and delivery system. The primary safety endpoint was in-hospital EDD device- or EDD procedure-related safety, defined as the hierarchical composite of cardiovascular mortality, major stroke disability, life-threatening (or disabling) bleeding, distal embolisation (non-cerebral) from a vascular source requiring surgery or resulting in amputation or irreversible end-organ damage, major vascular or access-related complications, and need for acute cardiovascular surgery. All endpoints were defined and adjudicated according to Valve Academic Research Consortium (VARC) recommendations by an independent committee which included a qualified cardiac surgeon, an interventional cardiologist and a vascular neurologist14.
The study included a powered secondary efficacy endpoint of presence of new embolic lesions detected by DW-MRI from pre-procedure to post procedure, compared with published historical controls. Other secondary endpoints included procedure success (successful device performance in the absence of primary safety endpoint component events), number and volume of new embolic lesions by DW-MRI, number of emboli detected by procedural transcranial Doppler ultrasound (TCD) monitoring, total procedure and EDD deployment time, cognitive evaluation, and overall (TAVR+EDD) major adverse cardiovascular and cerebrovascular events (MACCE) in hospital and at 30 days according to VARC definitions (hierarchical composite of all-cause mortality, major stroke, life-threatening [or disabling] bleeding, acute kidney injury [stage 3, including renal replacement therapy], periprocedural myocardial infarction, major vascular complication, and repeat procedure for valve-related dysfunction)13. All adverse events were adjudicated by an independent clinical events committee (CEC; Yale Cardiovascular Research Group, Yale University, New Haven, CT, USA).
DIFFUSION-WEIGHTED MAGNETIC RESONANCE IMAGING
DW-MRI of the brain was performed up to 21 days pre-procedure and at 4±2 days post procedure according to a standardised image acquisition protocol. DW-MRI images were reviewed and analysed using validated qualitative and quantitative methods (Vitrea Version 6.3.2; Toshiba America Medical Systems, Tustin, CA, USA) at an independent core laboratory (Global Institute for Research, Richmond, VA, USA) by a neuroradiologist blinded to clinical characteristics and temporal sequence. All lesions were adjudicated by a second imaging physician. Axial DW-MRI images and corresponding apparent diffusion coefficient (ADC) maps, as well as corresponding T2-weighted images, were reviewed for the presence of lesions with high signal intensity on DW-MRI. Acute ischaemic lesions were defined as areas of high signal intensity on DW-MRI with corresponding areas of low signal intensity on the ADC maps. Corresponding T2-weighted images were also reviewed for T2 shine-through. Coronal and sagittal image reformats were reviewed to determine craniocaudal extent and size of lesions. For each patient, the total number of lesions at pre- and post-procedure time points was recorded and the total number of new lesions calculated. The volume of each acute ischaemic lesion on DWI images was calculated using the “ABC/2” method. Within each two-dimensional axial slice, the long-axis diameter and the short-axis diameter of each lesion was manually measured using electronic calipers. For lesions that were only seen in a single axial slice, the measured long-axis diameter was “A”, short-axis diameter was “B”, and “C” was the slice thickness. When a lesion was seen in multiple connecting slices, the long- and short-axis diameters were measured in each slice. The greatest long-axis diameter and the greatest short-axis diameter were taken as “A” and “B” for the volume calculations, and “C” was computed as “slice thickness+interslice gap” x number of slices where the lesions were seen. Individual lesion volumes were summed across each patient to yield total lesion volume.
TRANSCRANIAL DOPPLER ULTRASOUND
Periprocedural TCD monitoring was performed for the duration of the procedure at suitably equipped sites by a single operator using a consistent protocol and equipment (Doppler-Box™ system [DWL, Compumedics, Singen, Germany] with a Lam Rack headset and two multi-frequency probes [2.0/2.5 MHz] connected to a dedicated laptop with QLab 2.10 software for analysis [Philips Healthcare, Best, The Netherlands]). After anaesthesia induction, Doppler probes were mounted bilaterally to the subject’s head and the middle cerebral artery (MCA) was insonated continuously until final withdrawal of all catheters. The recording session was divided and analysed by procedural stage: 1) before EDD placement; 2) positioning of the EDD delivery system; 3) deployment of the EDD; 4) TAVR procedure and retrieval; and 5) removal of the EDD and delivery system. The software automatically marked high-intensity transient signals (HITS) indicative of emboli, differentiated them from artefact, and classified them as solid or gaseous.
STATISTICAL ANALYSIS
The primary analysis was conducted in the “intention to treat” population. Continuous variables are presented as mean±standard deviation. Binary variables are described as frequencies and percentages. The hypothesis of the powered secondary efficacy endpoint is that TAVR with the EDD is superior to an objective performance criterion (OPC) with regard to the proportion of patients who develop new post-procedure cerebral ischaemic lesions as assessed by DW-MRI. The OPC was set at 76% based on historical data from five studies of patients undergoing unprotected TAVR, chosen based on similar patient populations and DW-MRI acquisition time points (Table 1)8,9,15-17.
A sample size of 28 subjects with paired DW-MRI data would provide 90% power to demonstrate superiority of TAVR with the EDD to the OPC for a two-sided α=0.05, assuming a 40% reduction in the proportion of subjects developing new lesions. The protocol allowed continued enrolment of up to 60 subjects to account for loss to follow-up or contraindication to post-procedure DW-MRI (e.g., permanent pacemaker implantation).
Results
SUBJECT AND PROCEDURAL CHARACTERISTICS
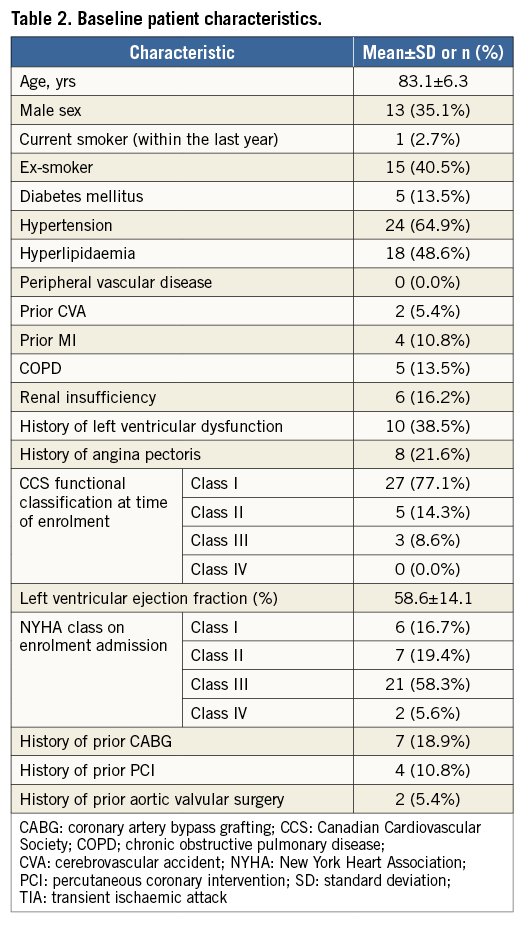
A total of 37 subjects were enrolled at six sites in Europe and Brazil. The study population is representative of patients meeting current indications for TAVR, with a mean age of 83.1±6.1 years, frequent comorbidities (smoking, hypertension, and hyperlipidaemia), and severe functional limitations (58.3% NYHA Class III) (Table 2).
A total of 42 valves were implanted in 37 patients (three valve-in-valve, one valve-in-valve-in-valve). The CoreValve transcatheter aortic valve was used in 64.3% (27/42) of cases and the SAPIEN transcatheter heart valve in 35.7% (15/42), all via the transfemoral approach. Balloon valvuloplasty was performed before TAVR in 76% (28/37) of cases. TAVR implantation was successful in 97.3% (36/37) of subjects; one subject required urgent conversion to surgical aortic valve replacement after failed implantation of two TAVR devices complicated by severe aortic insufficiency. Mean total procedure time was 91.0±25.1 minutes.
EDD PERFORMANCE
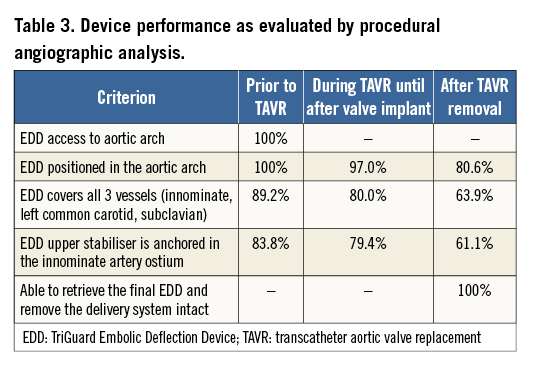
A total of 41 EDD devices were used in 37 patients; four patients had two EDD devices used. In two subjects in whom two devices were used, neither device could be oriented correctly and anchored in the innominate artery. In the third subject, the first EDD could not be oriented correctly, but a second EDD was successfully placed. The fourth subject underwent attempted placement of three consecutive TAVR prostheses: for each of the first two attempts, an EDD was successfully positioned but displaced and removed following retrieval of the valve. In all cases, the EDD and delivery sheath were able to access the aortic arch, be deployed, and be retrieved intact (41/41). No obstruction to cerebral blood flow or interference of the EDD with balloon aortic valvuloplasty or TAVR was reported. The device was successfully positioned to cover all three cerebral inflow vessels prior to passage of the TAVR catheter in 89.2% (33/37) of cases, remained in position through prosthetic valve deployment and implantation in 80.0% (28/35) of cases (primary protocol-defined performance endpoint), and remained in proper position after removal of the TAVR delivery system in 63.9% (23/36) of cases (Table 3). Therefore, the primary performance endpoint of device success was met in 80% (28/35) of cases. Procedure success (successful device performance in the absence of in-hospital procedure safety events) was 72.2% (26/36), and mean device deployment time was 13.3±11.6 minutes.
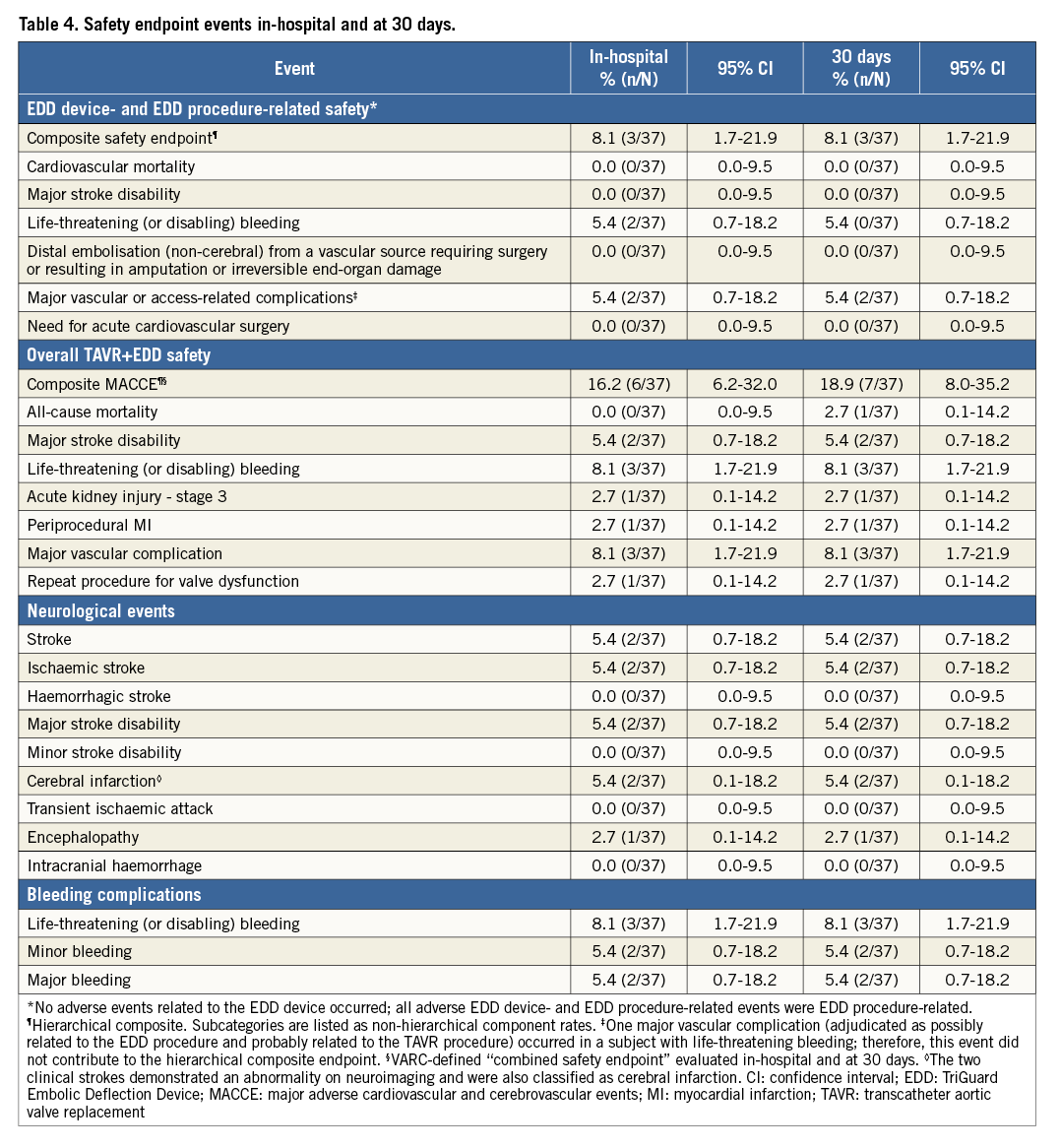
EDD SAFETY
The overall primary hierarchical composite safety endpoint of in-hospital EDD device- or EDD procedure-related safety occurred in 8.1% (3/37) of subjects, including two cases of life-threatening or disabling bleeding (rectus sheath haematoma, epidural haematoma) and one major vascular complication (femoral access-site oozing necessitating blood transfusion ≥4 units). All three events were unrelated to the EDD (i.e., the filter itself) and related or possibly related to the EDD procedure (i.e., vascular access). No additional EDD-related events occurred at 30-day follow-up.
The overall (TAVR+EDD) hierarchical composite in-hospital MACCE endpoint (VARC-defined) occurred in 16.2% (6/37) of subjects (two major stroke disabilities, two life-threatening bleeds, one periprocedural myocardial infarction, and one major vascular complication). One stroke occurred the day following urgent surgical conversion after a failed TAVR implant, and the second occurred in a patient whose procedure was complicated by loss of ventricular capture from a temporary pacing lead in the setting of complete heart block, requiring prolonged cardiopulmonary resuscitation. The two strokes were not EDD-related by independent CEC adjudication. The major vascular complication was a thoracic aortic dissection in the same patient who underwent two failed TAVR attempts with urgent conversion to surgery. The dissection occurred intraoperatively following manual manipulation and removal of the TAVR device. It was repaired surgically and the patient recovered. At 30 days, the VARC-defined overall MACCE endpoint was 18.9% (7/37); the additional event was a non-cardiovascular death in a patient who developed pneumonia after hospital discharge. Safety endpoint event rates are summarised in Table 4.
CEREBRAL ISCHAEMIC LESIONS
Interpretable post-procedure DW-MRIs were available in 28 subjects. In the remaining nine subjects, post-procedure imaging was not completed due to contraindications to DW-MRI (permanent pacemaker implantation or unstable clinical status) in six cases (includes the two patients with disabling stroke), and consent was withdrawn in three cases. All patients had baseline DW-MRI; however, two subjects with post-procedure DW-MRIs did not have interpretable baseline images. In these subjects, we imputed zero lesions at baseline, an approach that assumes a “worst case scenario” in terms of device efficacy by maximising the number of lesions that are counted as new post-procedure lesions. DW-MRI data are presented in Table 5. The proportion of patients with new ischaemic lesions was not significantly different from the performance goal derived from historical controls of unprotected TAVR (82% vs. 76%; p=NS). Average new single lesion volume was non-normally distributed; median was 0.03 (0.01-0.06) cm3. Median new single lesion volume was 80% lower compared to historical data (0.15 cm3). Average maximum single lesion volume was also not normally distributed; median was 0.06 (0.002-0.2) cm3. Median number of new ischaemic lesions was three (1.8-8.0). Median total lesion volume was 0.2 (0.03-0.4) cm3 and 34% lower than reported historical data (0.3 cm3). Among the 28 patients with analysable MRI, 17 patients had complete cerebral vessel coverage, 10 had incomplete coverage and one could not be adjudicated. Patients with complete coverage had fewer lesions (two vs. 8, p=0.012), and the median total lesion volume was 89% lower (0.05 vs. 0.45 cm3, p=0.016) as was the median single lesion volume (p=0.004) (Figure 2). Lesion distribution was slightly biased to the left cerebral circulation (60% vs. 40%); the most commonly affected vascular territory was the MCA (47%), followed by the posterior cerebral artery (22%), posterior inferior cerebellar artery (17%), anterior cerebral artery (13%), or other (2%).
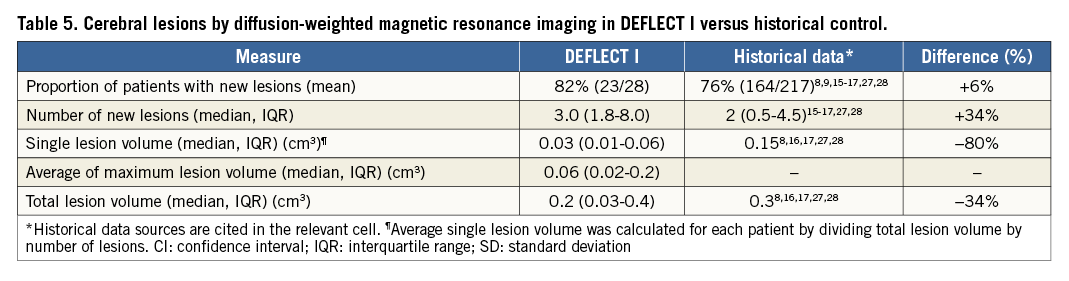
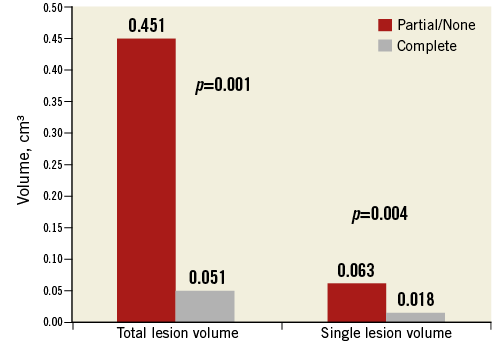
Figure 2. DW-MRI results in DEFLECT I. Complete versus incomplete cerebral vessel coverage.
COGNITIVE OUTCOMES
Neurocognitive outcomes were evaluated in subjects with complete MoCA data at all three time points, and for whom post-procedural DW-MRI data were available (n=21). MoCA scores improved monotonically from the baseline to post procedure and 30 days (p-value for linear trend=0.030) (Figure 3). Despite the mean increase in performance over time, variability in MoCA test scores increased with each visit. Formal testing of the normality of the distribution of MoCA scores (Shapiro-Wilk [S-W] test) revealed a normal distribution at baseline (S-W statistic (21)=0.949, p=0.333) and post procedure (S-W statistic (21)=0.942, p=0.241); however, the score distribution deviated from normality at the 30-day assessment (S-W statistic (21)=0.905, p=0.041). Based on an impairment threshold score of 26, an exploratory comparison of impaired (n=13) versus unimpaired subjects (n=7) at 30 days was performed relative to the total volume of new post-procedure DW-MRI lesions and controlling for baseline MoCA score. The impaired group had a numerically higher median total lesion volume (163.18 cm3) compared with the unimpaired group (130.05 cm3); this difference was not statistically significant (p=0.734). Subjects classified as impaired at 30-day follow-up had lower MoCA scores at screening (24.50±2.94 vs. 25.71±2.69, p=0.006) and at the discharge visit (21.25±2.86 vs. 28.38±1.77, p<0.001) than unimpaired subjects. The two groups did not differ in age (p=0.394) or sex distribution (p=0.052).
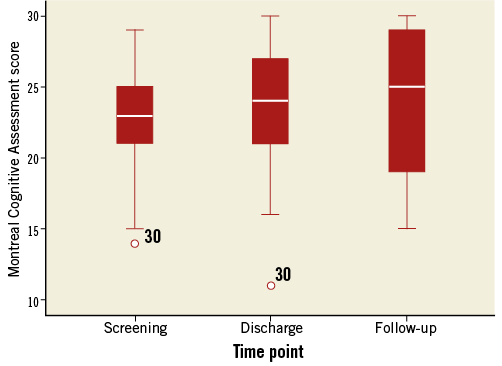
Figure 3. Cognitive outcomes. Distribution of Montreal Cognitive Assessment scores at the three clinical visits.
TRANSCRANIAL DOPPLER ULTRASOUND
TCD monitoring was performed in 25 subjects and interpretable in 17 subjects. The total count of MCA HITS for each step of the procedure (sum of right and left sides) is reported in Figure 4. HITS were detected during all steps of the procedure (mean 836±134; 186±29 solid and 650±107 gaseous). TAVR-specific procedural steps accounted for >80% of both total (670±158) and solid HITS (150±34). Of TAVR-related steps, HITS were most numerous during deployment of the valve prosthesis (25.4%), wire and catheter crossing of the aortic valve (20.8%), balloon valvuloplasty (11.5%), and positioning of the valve prosthesis in the native valve (9.8%). EDD-related steps accounted for 19.8% of total (166±68) and solid HITS (37±16). Of these, the vast majority (82% of total and 79% of solid HITS) occurred during introduction and positioning of the EDD delivery system, comparable to the number of HITS observed during guidewire and pigtail catheter passage over the aortic arch. Actual deployment of the EDD resulted in a relatively small number of HITS (20.4±7.6 total, 5.4±3.8 solid) (Figure 4).

Figure 4. Transcranial Doppler ultrasound results. Total number of solid and gaseous middle cerebral artery high-intensity transient signals (HITS) by procedure stage.
Discussion
The results of the DEFLECT I study provide preliminary evidence for the performance and safety of the first-generation TriGuard EDD, and establish proof of concept for potential efficacy in limiting embolic brain lesion volume compared to historic controls as well as among those patients in DEFLECT I with complete versus incomplete cerebral coverage. On the basis of DEFLECT I, the TriGuard EDD obtained CE mark approval, and these results warrant further randomised evaluation to establish clinical benefit.
The device was successfully deployed and retrieved in all cases, and could be positioned to provide complete coverage of all three cerebral vessel take-offs through TAVR valve implantation in 80% of cases - the period during which the majority of cerebral embolic events have been demonstrated to occur6,7. Importantly, the majority of TriGuard EDD performance failures occurred during the first two cases at each site, emphasising the importance of operator experience. The TriGuard EDD maintained position until after retrieval of the TAVR delivery system in 64% of cases. The incremental benefit of continued neuroprotection during TAVR system retrieval is unclear. Previous studies of TCD (confirmed in the current study) demonstrate that the majority of microembolisation occurs during TAVR prosthesis positioning and deployment, with relatively little subsequent generation of emboli6,7. Our study confirms that the majority of solid embolisation occurs prior to or during TAVR deployment; however, 4.7% of TCD HITS occurred during TAVR delivery system removal. Therefore, optimal embolic protection should include complete procedural coverage, including removal of the TAVR delivery system. Importantly, positioning and removal of the EDD resulted in a minimal increment of solid HITS beyond background levels (Figure 4).
Use of the TriGuard EDD device as an adjunct to TAVR was associated with an increment of access-site adverse events. No adverse events related to the EDD itself were reported - the composite investigational device-related and investigational procedure-related MACCE rate of 8.1% (3/37) was driven by bleeding and vascular complications related to the 9 Fr delivery system inherent to transfemoral catheter-based procedures. Two disabling strokes occurred in DEFLECT I, but neither was related to the investigational device or procedure as determined by independent adjudication, and neither stroke was probably preventable by procedural neuroprotection (one occurred one day post procedure following urgent surgical conversion of failed TAVR, the second following cardiopulmonary resuscitation).
Major stroke occurs in 3.8-5% of subjects in randomised TAVR trials within 30 days17-19, approximately half of which are periprocedural3. While neuroprotection could have a substantial impact on the periprocedural stroke rate, complete elimination of stroke is unlikely to be achievable due to the heterogeneous nature of stroke in this high-risk patient population (e.g., new-onset atrial fibrillation, hypoperfusion, thrombosis of the recently implanted valve, surgical conversion of failed TAVI)20-22. Overall, complication rates of TAVR with the EDD (including bleeding and vascular complications) were similar to those observed during unprotected TAVR in comparable patient populations18,19, indicating that adjunctive use of the EDD does not pose significant incremental risks. Nevertheless, next-generation EDD device development is focused on lower profile with a lower 7 Fr access sheath size. No distal non-cerebral embolisation was reported in this study, supporting the safety of deflection as a mechanism of cerebral embolic protection. The use of the EDD itself was associated with relatively few solid emboli on TCD; however, the potential additive embolic risk of any cerebral protection device requires close scrutiny.
Numerous studies have demonstrated the cognitive implications of new cerebral ischaemic lesions on DW-MRI following cardiac procedures23-25. Compared with a performance goal based on historical data, use of the EDD did not result in a reduction in the presence of new embolic lesions detected by DW-MRI. However, analysis of other DW-MRI lesion parameters found that median total lesion volume was 34% lower, and median single lesion volume was 80% lower than might be expected based on historical data from unprotected TAVR. Furthermore, in the DEFLECT I population, there was an 89% lower median total lesion volume among the 80% of patients with complete cerebral coverage compared to those with incomplete coverage. The putative mechanism of this effect is that, while small debris may continue to pass through the EDD filter’s 250 µm pores, the largest (and potentially most harmful) emboli are prevented from reaching the cerebral circulation. The next-generation EDD has further reduced the filter pore size to 120 µm. Thus, while EDD protection may not eliminate stroke due to its heterogeneous aetiology, the potential benefit of EDD in reducing lesion volume is expected to improve cognitive function. Prior studies have shown that mean single lesion volume after endovascular procedures is associated with dementia and cognitive changes26, and total lesion volume is predictive of clinical outcome after stroke27,28. Thus, reducing lesion volume may improve neurocognitive outcomes. Our study indicates that cognitive function improves over time after TAVR, possibly due to a combination of practice effects on the screening instrument and increased blood flow to the brain. While this study was underpowered to detect a statistically meaningful result, a possible association between cerebral ischaemic lesion volume and cognitive impairment at 30 days was noted.
Limitations
The DEFLECT I study is a non-randomised safety and performance study in a limited number of patients. The results are intended to provide proof-of-concept and preliminary data to guide more rigorous randomised evaluations. Safety endpoint comparisons to unprotected TAVR are limited by the small sample size, lack of an active control group, and advances in the practice of TAVR over time. For efficacy endpoints, the study was powered to detect a potential reduction in the incidence of new lesions; all other DW-MRI results should be considered hypothesis-generating. In particular, comparisons to historical lesion volumes should be interpreted with caution: published studies have used a variety of analysis methodologies and reported a wide range of single lesion and per-patient volumes. Loss to follow-up despite best efforts in this study (24% loss to DW-MRI follow-up at 4±2 days, and 43% loss to complete cognitive and neuroimaging follow-up) highlights the challenges of implementing detailed data collection in the complex, comorbid, and elderly TAVR patient population. Importantly, DW-MRI was not performed in the two patients with major stroke disability, probably underestimating lesion volumes by ITT; however, the strokes were adjudicated as non-EDD-related by an independent CEC. Regarding cognitive outcomes, no universally accepted instrument exists to quantify the impact of cardiovascular procedures. The MoCA was adopted in this study to expand sensitivity to detect clinical changes below the threshold of clinical neurological scales; however, this instrument’s ability to detect meaningful cognitive impairment has not been validated against a full neuropsychological battery in this patient population.
Conclusions
The DEFLECT I trial demonstrates that the first-generation TriGuard EDD is a safe adjunct to TAVR, and provides complete 3-vessel cerebral embolic protection through valve implantation in 80% of cases. The proportion of patients who developed new post-procedure ischaemic lesions was not significantly different from a performance goal based on historical data from unprotected TAVR; however, based on exploratory MRI analyses, the TriGuard EDD appears to reduce total ischaemic brain lesion volume. These preliminary results warrant further evaluation of safety and effectiveness in a planned randomised controlled trial of the next-generation TriGuard device.
| Impact on daily practice The prospective, multicentre DEFLECT I study demonstrated that use of the first-generation TriGuard Embolic Deflection Device during TAVR is safe. It was successfully placed in 80% of cases. This establishes proof of concept for this device’s potential efficacy in limiting embolic brain lesion volume. The ability of the TriGuard Embolic Deflection Device to reduce total cerebral ischaemic burden and improve clinical benefit warrants additional evaluation. |
Acknowledgements
Participating centres: Michael Mullen, MD, FRCP; University College of London Hospitals, NHS Foundation Trust, The Heart Hospital; Jan Kovac, MUDR, FACC, CCST, MD; University Hospitals of Leicester, NHS Foundation Trust, Glenfield Hospital; David Hildick-Smith, MD, FRCP, FSCAI; Brighton and Sussex University Hospital; Andreas Baumbach, MD, FRCP, CESC; University Hospitals of Bristol NHS Foundation Trust; Peter den Heijer, MD, PhD; Amphia Ziekenhuis Molengracht Breda; Alex Abizaid, MD, PhD, FACC; Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil.
Funding
This study was sponsored by Keystone Heart, Ltd. A. Baumbach is supported by the NIHR Bristol Cardiovascular Biomedical Research Unit.
Conflict of interest statement
A. Brickman and A. Lansky serve on the scientific advisory board for Keystone Heart; P. Margolis is a consultant and shareholder in Keystone Heart and S. Voros is a shareholder in Keystone Heart. The other authors have no conflicts of interest to declare.

