Abstract
Aims: To report our first-in-man experience with a new cerebral embolic deflection device (SMT Embolic Deflection Device) during transcatheter aortic valve implantation (TAVI). A significant number of strokes and brain infarcts are caused by embolisation of atherosclerotic material, clots and other debris during various phases of invasive cardiac procedures, especially TAVI. The application of a temporary filter in the aortic arch averting dislodged emboli from entering the cerebral circulation might prevent this.
Methods and results: In 15 patients (mean age 79 years) with severe aortic stenosis undergoing percutaneous transfemoral or transapical aortic valve implantation, the SMT Embolic Deflection Device was advanced utilising the contralateral femoral artery access using a 9 Fr delivery sheath. Once deployed in the aortic arch, a porous membrane shields the supraaortic-cerebral trunks by deflecting emboli away from the cerebral circulation. Embolic material is not contained or removed by the device. A 6 Fr pigtail catheter can be used through the same sheath throughout the whole procedure. Brain diffusion weighted (DW)-MRI was obtained in 10 patients before and at 4 days after (±2 days) the procedure and retrospectively compared to 20 patients previously undergoing TAVI without a protection device. Successful placement of the embolic protection device was achieved in all patients. Additional procedural time due to the use of the device was 7 min (±2 min). There were no procedural complications. No patient developed new neurological symptoms or clinical findings of stroke except one patient who suffered from a transient ischaemic attack (TIA) two days after the procedure. DW-MRI showed 3.2 new cerebral lesions per patient, compared to 7.2 new lesions per patient in the group without SMT filter.
Conclusions: In this first-in-man experience, the feasibility of a new embolic deflection device is demonstrated. Larger randomised, prospective studies are required to confirm these findings and prove safety and efficacy by reducing the incidence of cerebral embolism and stroke after TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as a treatment option for inoperable or high-risk surgical patients with severe aortic stenosis. While TAVI reduces 1-year mortality as compared to conservative treatment and reduces perioperative morbidity as compared to surgery with similar mortality outcomes, there is an increased risk of neurological complications. In high-risk surgical candidates in the PARTNER trial, TAVI was associated with an approximately two-fold increased incidence of stroke or transient ischaemic attack (TIA) (5.5% vs. 2.4%, p=0.04) compared with surgical aortic valve replacement (SAVR) at 30 days1. In contrast to percutaneous treatment of coronary artery disease which has managed to achieve a very low incidence (0.22%) of periprocedural stroke2, the percutaneous treatment of a diseased aortic valve clearly still comes at a higher price. The passage of bulky devices through a diseased aorta3 and the aggressive manipulation of a stenotic, calcified aortic valve may cause embolisation of atherosclerotic material, clots and other calcified debris. Indeed, as already previously demonstrated during diagnostic angiogram as work up for SAVR, left catheterisation with retrograde crossing of the aortic valve has been associated with a higher incidence of new focal diffusion-imaging abnormalities on magnetic resonance imaging (MRI) as compared to catheterisation without passage of the valve, with new lesions in 22% of patients (suggesting new cerebral embolic events), and a 3% rate of clinically relevant neurological deficits4. A study utilising transcranial Doppler ultrasound during TAVI demonstrated the occurrence of cerebral microemboli in each of 52 patients, mainly during direct manipulation of the diseased valve and crushing of the leaflets during implantation5. Only a minority of these patients suffered from clinical stroke or TIA, but new foci of restricted cerebral perfusion on diffusion weighted (DW)-MRI are reported in 58% to 91% of TAVI patients6-10. The clinical impact of these new, asymptomatic DW-MRI lesions is uncertain but, in epidemiological studies, their presence has been associated with declines in cognitive and physical function, frailty, development of dementia and an increased risk of subsequent stroke11. Embolic protection devices have already demonstrated their efficacy in the interventional treatment of saphenous vein graft disease12 and carotid lesions13. The application of a similar concept during TAVI could minimise both clinical and sub-clinical cerebral emboli.
We report our first-in-man experience with the SMT Embolic Deflection Device (SMT Research and Development Ltd., Herzliya, Israel), a retrievable low-profile filter, designed for percutaneous delivery to the aortic arch, for the prevention of stroke caused by emboli originating in the heart and aorta.
Methods
Patients
The SMT filter was used during aortic valve intervention in 15 patients with symptomatic severe aortic stenosis. These patients were formally discussed in the heart team and considered inoperable or at high risk for SAVR due to age, a high logistic EuroSCORE, porcelain aorta, malignancy, frailty or severe comorbidities. Informed consent for use of the SMT device was obtained.
SMT Embolic Deflection Device (Figure 1 and Figure 2)
The SMT Device is a sterile, biocompatible filter, implanted in a transcatheter procedure, through a needle puncture in either common femoral artery, and is located under fluoroscopy in the aortic arch. While the filter does not block or decrease normal blood flow to the brain via the aortic branches and vertebral artery, it diverts emboli/particulate matter downstream, where they can be treated effectively or probably cause less harm, although clinical impact on kidney and other end-organ function has to be further established. The SMT device is made of fine nitinol #1 (nickel titanium alloy) wires, which exhibit superelasticity so that the filter can be crimped into an 8 or 9 Fr sheath, the latter providing the possibility to use simultaneously a 6 Fr pigtail through the same sheath, hence avoiding additional groin punctures. Upon deployment the filter unfolds and regains its original shape.
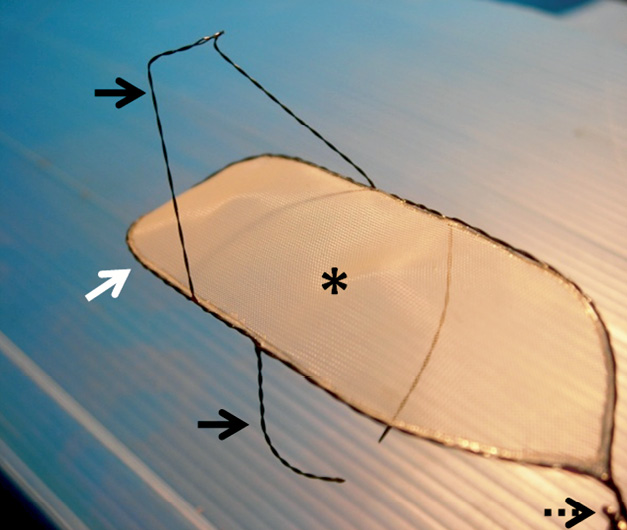
Figure 1. MT embolic deflection device. The SMT device consists of 5 functional parts: a dual wire nitinol frame (white arrow), a thin Nitinol mesh (asterisk), an upper and lower stabiliser (black arrows) and the tail end (dotted arrow) (see text for further detail).
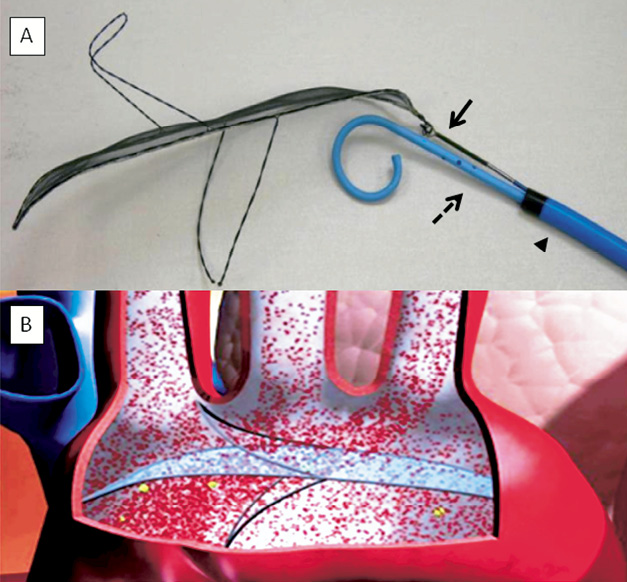
Figure 2. A) MT device attached to plunger. The SMT filter upon deployment, attached to the plunger of the delivery system (arrow) inside a 9 Fr sheath (arrowhead) and with simultaneous advancement of a 6 Fr pigtail catheter (dotted arrow). B) Animated illustration of a deployed SMT device in the aortic arch.
The SMT Device consists of 5 functional parts (Figure 1 and Figure 2):
1. A dual wire nitinol frame that anchors the device in the desired location in the aortic arch.
2. A thin nitinol mesh designed to allow blood flow through, while diverting clinically significant emboli towards the descending aorta.
3 and 4. Two stabilisers that facilitate the positioning of the filter. They lock into position by retrograde traction and gradual release from the delivery shaft. The filter is anchored in the aortic arch by the upper stabiliser in the innominate artery ostium which prevents filter retrograde migration and by the lower stabiliser that push the filter in apposition with the upper wall of the aortic arch.
5. The tail end (distal from the heart) of the SMT filter is a connection, by which the SMT filter remains securely attached to the plunger (“pusher”) during the procedure.
The filter is coated to shield the blood from the underlying medical device material. The chemical and physical properties of the coating reduce the likelihood for blood components to adhere and activate, thus reducing the formation of thrombus or emboli. Interestingly, the SMT filter was originally developed as a permanent surgical implant for patients with high risk of stroke by cardiac emboli, however subsequently technically modified for temporary transcatheter implants.
Delivery system
The delivery system includes the following components:
The 9 Fr delivery sheath (Cook Medical, Bloomington, IN, USA) is a 75 cm long catheter, curved at the distal end. The direction of the curve is opposite to the sidearm fitting, which allows flushing the sheath even when a catheter or the plunger is inside.
The dilator is a matching tube (also part of the Cook Medical set) with a 1 mm lumen (for a guidewire). It fits snugly into the 9 Fr delivery sheath and has a female luer connector at the proximal end, for rinsing.
The loading tube with a female luer hub at the proximal end is used for crimping and loading the SMT filter. It connects to the delivery sheath by being inserted (the smooth distal end) into the septum hub. It is attached to a 3-way Y-connector homeostasis valve at its proximal end to prevent blood dripping and allow access to the delivery sheath.
The crimper is a funnel that enables easy loading of the SMT device into the cartridge. It mounts on the distal end of the cartridge prior to loading, and it is removed after loading.
The plunger (delivery shaft) is a flexible wire used for advancing the filter from the cartridge into the delivery sheath and through it to the site of deployment. It is also used for retrieving the SMT filter back into the 9 Fr delivery sheath.
Aortic valve implantation
All TAVI procedures were routinely performed by a team, consisting of 2 interventional cardiologists, one cardiologist specialised in echocardiography, one cardiac surgeon, one anaesthetist and 2 specialised nurses. After deployment of the SMT filter (Figure 3), an Edwards SAPIEN (Edwards Lifesciences, Inc., Irvine, CA, USA) transcatheter aortic valve was implanted in 15 patients, who were all pretreated with aspirin and clopidogrel and received heparin during the procedure in order to maintain an activated clotting time above 250 ms. Nine patients were approached via the transfemoral way, the remaining 6 underwent a transapical procedure according to standard techniques14,15. The size of the valve was chosen according to echocardiographic estimates of aortic annulus diameter and varied between 23 and 26 mm. All procedures were done under general anaesthesia and with transoesophageal echocardiography guidance. The stenotic valve was predilated with an undersized balloon to facilitate valve implantation and subsequent valve deployment was accomplished during rapid pacing in the right ventricle. If necessary, additional post-dilation was performed in case of relevant paravalvular regurgitation. At the end of the procedure, the SMT filter was retrieved into the delivery sheath and removed.
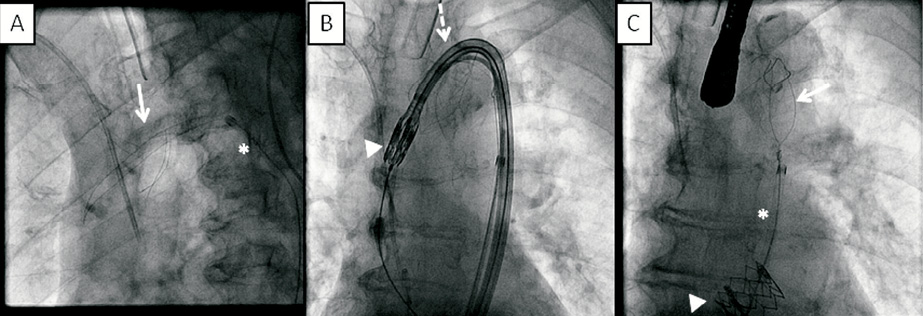
Figure 3. Fluoroscopic images of an SMT device in the aortic arch during transfemoral TAVI. The SMT filter (arrow) is deployed in the aortic arch at the beginning of the procedure through a 9 Fr delivery sheath (asterisk). Unhampered passage of the Edwards SAPIEN valve (arrowhead) through the aortic arch via the Retroflex delivery system (Edwards Lifesciences, Inc., Irvine, CA, USA) (dotted arrow). After successful aortic valve implantation (arrowhead), the SMT device (arrow) is retrieved in the 9 Fr delivery sheath (asterisk).
MRI
MRI scanning of the brain before TAVI and <1 week after was performed in 10 patients undergoing TAVI with cerebral protection device and retrospectively compared to 20 patients who had undergone TAVI without cerebral protection previously in our institution. Scanning was performed according to current standard practice. The MRI scan included a diffusion weighted imaging (DWI) scan sequence. Scans were scored by two blinded observers in consensus. New ischaemic lesions were defined as new areas of high signal intensity on the post-procedural DWI images. On each scan the number of lesions with high signal intensity on DWI images (representing ischaemic lesions) was counted.
Statistical analysis
Due to the observational single arm nature of this registry, only descriptive statistics has been performed. Data are presented as mean ±standard deviation if continuous or as number (percentage) if dichotomous.
Results
Baseline patient characteristics (Table 1)
The SMT device was used in 15 patients, 11 women and four men, with a mean age of 79.3 years (±9.9). Risk factors for stroke included previous stroke/TIA (5 patients), severe peripheral vascular disease (6 patients) with presence of a porcelain aorta in 3 patients, diabetes mellitus (4 patients), hypertension (10 patients) and dyslipidaemia (10 patients). Three patients underwent percutaneous coronary intervention at least 1 month before TAVI because of unstable angina or myocardial infarction and 3 patients had a history of CABG. One patient had a very poor left ventricular systolic function (ejection fraction <30%).
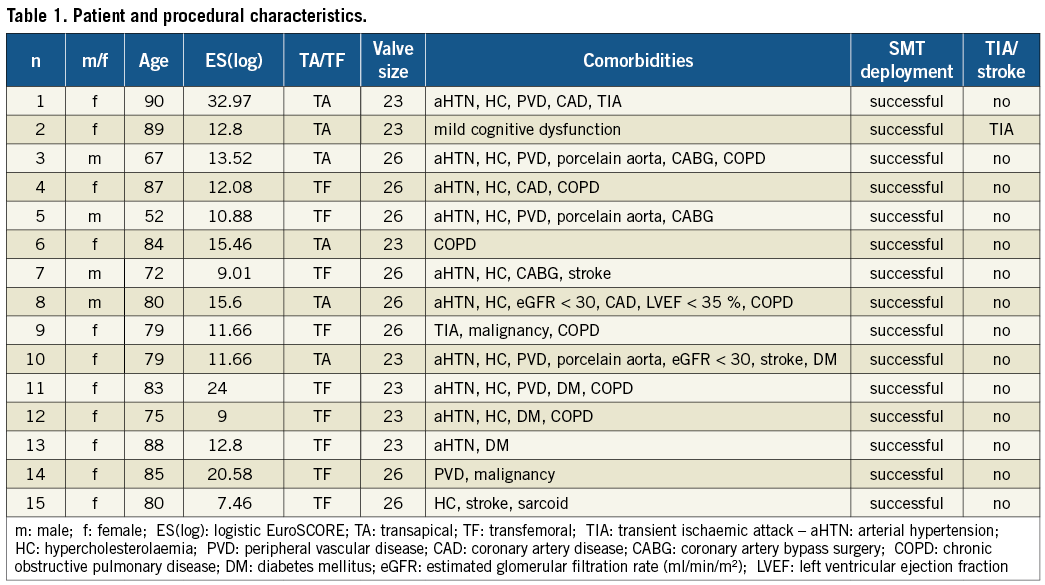
Procedural and clinical outcome (Table 1)
In all cases, the SMT filter was deployed without complications across the aortic arch via a 9 Fr femoral arterial sheath at the beginning of the procedure and withdrawn at the end of the procedure (demonstrated in Figure 3). No vascular or bleeding complications occurred at the femoral access site, which was closed successfully in all patients with an 8 Fr Angio-Seal closure device (St. Jude Medical, St Paul, MN, USA). Additional procedural time due to the use of the device was 7 min (±2 min). On the basis of fluoroscopic images, the device immediately after deployment seemed to cover the ostia of the three supraaortic trunks (brachiocephalic, left carotid and left subclavian) in all cases. In case of transfemoral TAVI, subsequent passage of pigtail, stiff wires, predilatation balloon and valve prosthesis was not hampered by the in situ device. During in-hospital follow-up, no patient developed new neurological symptoms or clinical findings of stroke except for one patient who suffered from a TIA 2 days after the procedure. No patient suffered from clinically relevant peripheral embolisation towards non-brain regions.
MRI results
MRI scanning of the brain in 10 patients with SMT filter showed on average 3.2 new DW lesions per patient, as compared to 7.2 new lesions per patient in the historical comparison group without SMT filter. Lesions occurred almost equally in left and right cerebral and cerebellar hemispheres in both treatment groups.
Discussion
Clinical and subclinical cerebral embolism during TAVI is a matter of concern and a drawback for the ubiquitous introduction of this revolutionary treatment. Alongside vascular access complications, it obviously represents one of the “weakest links” of TAVI for which the interventional community has to seek a solution. Indeed, while periprocedural clinical stroke and TIA probably only represent the tip, underneath is an iceberg of subclinical new cerebral lesions caused by TAVI which may be associated in the long term with accelerated cognitive and functional decline. Especially if TAVI has the potential to become a treatment for younger patients with aortic stenosis in the foreseeable future, this issue has to be tackled. While downsizing of valve delivery equipment16, increased operator experience and better patient selection may all contribute to a reduction in neurological complications, mechanical manipulation of a diseased and calcified aortic valve will still cause a shower of debris towards the brain which necessitates some kind of sentinel at the entrance of the cerebral circulation, allowing blood to pass but capturing or deflecting all other “unwanted visitors”. Several embolic protection/deflection devices (e.g., Embrella [Edwards Lifesciences, Inc., Irvine, CA, USA], Claret [Claret Medical, Inc. Santa Rosa, CA, USA]) for use during TAVI are currently being tested17. The SMT device is a retrievable low-profile filter designed for transfemoral percutaneous delivery at the level of the aortic arch to reduce the occurrence of stroke by deflecting emboli that occur during instrumentation of the heart, aortic root and branching vessels. It is made from nitinol (nickel titanium alloy) wires, which exhibit super elasticity so that the filter can be crimped into a 8 Fr calibre catheter and regains its original shape upon deployment. A potentially major advantage of the SMT filter over the Embrella and Claret devices is the fact that the last two cover only the innominate and right carotid arteries, while the SMT filter covers all the supraaortic trunks. Possible drawbacks are that it is, like the Embrella, an embolic deflection instead of capture device, and it is larger, necessitating femoral access, while both Claret and Embrella devices can be delivered through a 6 Fr sheath.
In 15 patients, of whom 6 were known to have peripheral vascular disease including 3 with a porcelain aorta, we managed to position the device without complications in the aortic arch, where it shielded the ostia of all major arteries arising from the aortic arch according to the fluoroscopic images. The average total procedural time was only lengthened by 7 (±2) minutes, indicating the ease and user-friendliness with which the device can be deployed. Apart from one patient who suffered a TIA 2 days after TAVI, all patients remained free from clinical neurological events or symptomatic peripheral embolism. Although not the aim of this small feasibility study, we found that the number of new brain lesions on DW-MRI, which may prognosticate future cognitive dysfunction, was numerically reduced by more than half as compared to an historical group undergoing TAVI without the SMT deflection device at our centre. Whether this reduction is associated with better long-term neurological performance remains to be proven in larger, prospective randomised trials comparing protection versus no protection. Interestingly, new lesions were found substantially equally distributed in the whole central nervous system, in both left and right cerebral and cerebellar hemispheres. This finding supports the idea that protection should be provided to all cerebral vessels, whereas partial protection may be insufficient.
Study limitations
Due to the low number of patients, this study can only be considered as “feasibility-test”, paving the way first for a safety CE approval study (ongoing) and subsequently for a large, prospective randomised trial with enough power to demonstrate clinical efficacy and safety. While delivery and deployment of the deflection device was successful in all our patients, certain aortic arch anatomies may be inaccessible for the device or render efficacious deployment impossible, with persistent access for debris towards the cerebral circulation. It can be expected that, with the use of aortic protection devices, preprocedural computed tomography imaging will become even more important for the planning of TAVI procedure, not only to assess the aortic valve (calcifications, measurement of the annulus) and the ileofemoral system (calcifications, tortuosity), but also to evaluate the aortic arch and its potential to harbour a specific protective filter and meanwhile facilitate easy valve-device crossing.
The patients in our study did not undergo formal neurological assessment pre- and post-procedure, implying that subtle neurological deficits after TAVI may be missed. Future studies investigating the use of TAVI protection devices will require pre- and post-procedural patient evaluation by a neurologist and long-term patient follow-up to understand the potential clinical impact of a reduction in DW-MRI cerebral lesions.
Finally, the SMT device is a deflection device that redirects particulate matter away from the brain towards the descending aorta and peripheral organs. Although in our study this was not associated with any obvious clinical events, larger studies are needed to understand its potential negative clinical impact on kidney, liver or gastrointestinal function.
Conclusion
The use of the transfemoral SMT deflection device during transfemoral or transapical TAVI seems feasible and is associated with less cerebral embolism on DW-MRI in this small proof-of-concept study. Larger, randomised trials are required to confirm these findings and to understand the clinical risk reduction of (silent) cerebral embolism and stroke.
Conflict of interest statement
P.R. Stella is member of the scientific advisory board of SMT Medical. All other authors have no conflict of interest.

