In this chapter of Tools and Techniques, embolic protection devices in transcatheter aortic valve implantation (TAVI) are discussed. The following is a summarised overview of this technique. The complete, unabridged version with images is available online at: http//:www.pcronline.com/eurointervention/85th_issue/45.
Background and indications
As the technique of transcatheter aortic valve implantation (TAVI) is maturing and its application broadening, a reduction of TAVI-related complications is crucial. TAVI has proven to be superior to medical therapy in inoperable patients with aortic stenosis (AS) and at least as effective as surgical aortic valve replacement (SAVR) in AS patients at high risk of perioperative complications and mortality1,2. The first randomised trials seemed to suggest that clinically overt neurological events complicated TAVI in comparison to medical therapy or the more invasive SAVR1,2. Recently, the randomised US pivotal CoreValve trial refuted this premature notion. In a carefully designed trial setting encompassing neurologists who assessed patients before and after aortic valve replacement, there was no difference in clinical neurological events between TAVI and SAVR3. Nevertheless, TAVI implies: 1) the use of large-bore catheters, 2) passage through an aged and diseased aortic arch and ascending aorta, 3) the crossing of a calcified and degenerated aortic valve, and 4) positioning and deployment of a transcatheter valve within the diseased native aortic valve. Brain magnetic resonance imaging (MRI), transcranial Doppler and histopathology studies have revealed that cerebral embolisation is inherent to TAVI4-8. Although most TAVI cases seem uneventful from a clinical neurological perspective, silent brain ischaemia and defects occur in up to 80% of patients9. These silent brain lesions and microinfarcts may not be so harmless after all as an association with premature neurocognitive impairment seems to have been established10-12. Especially in patients with a longer life expectancy, these events may thus become clinically and socially relevant. Cerebral embolic protection devices may reduce intraprocedural cerebral embolisation.
Devices
Two fundamentally different designs are on the market: deflectors and filters. The Embrella (Edwards Lifesciences, Irvine, CA, USA) (Figure 1, Moving image 1) and the TriGuard™ (Keystone Heart Ltd, Caesarea, Israel) (Figure 2, Moving image 2) are deployed along the outer curve of the aortic arch and provide (more or less) coverage of the brachiocephalic trunk, the left common carotid artery and more variably the left subclavian artery by deflecting embolised material into the descending aorta13-15. The Sentinel (Claret Medical Inc., Santa Rosa, CA, USA) contains two filters to be deployed in the brachiocephalic trunk and left common carotid respectively16 (Figure 3, Moving image 3). The safety and feasibility of embolic protection devices (EPD) is established, yet their clinical efficacy is as yet unsettled17. Recently presented data on EPD suggest a reduction in number and volume of new brain lesions after TAVI and subtle but favourable neurological outcomes.
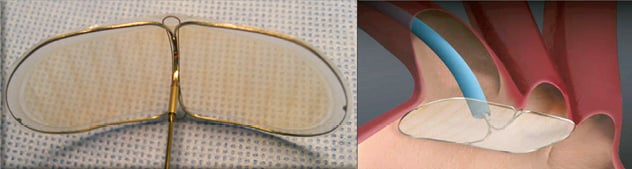
Figure 1. The Embrella Embolic Deflector system has an oval-shaped nitinol frame with a polyurethane membrane with 100 um pores. The frame has two opposing petals that cover the ostia of the brachiocephalic trunk and the left common carotid artery.
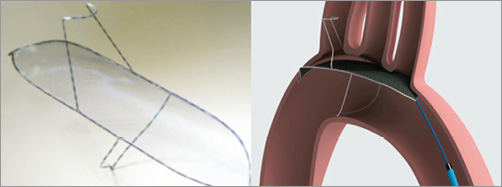
Figure 2. The TriGuard™ Embolic Deflection device has a nitinol frame that contains a nitinol mesh with 250 (and in the latest design 130) um pores and antithrombotic coating. It contains two stabilisers for optimal positioning and stability. It covers the ostia of the brachiocephalic trunk, the left common carotid artery and the left subclavian artery. An atraumatic stabiliser in the brachiocephalic trunk supports the position throughout the procedure.
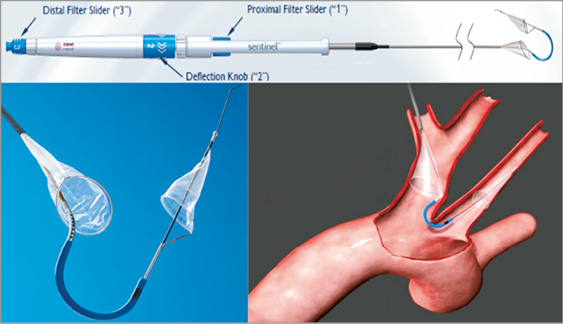
Figure 3. The Sentinel Dual Filter device consists of a steerable and rotatable catheter that contains two polyurethane mesh filters with 140 um pores mounted on a nitinol frame. One filter is deployed in the brachiocephalic trunk and the other in the left common carotid artery.
As noted above, the complete online version of this EuroIntervention Tools and Techniques topic will cover the following:
CURRENT EPD TECHNOLOGY INCLUDING IMPLANTATION TECHNIQUE
The Embrella and Sentinel devices, in principle, deal with three out of four extracranial contributory arteries leaving the left vertebral artery unprotected. They require 6 Fr radial access. The TriGuard aims to cover the entire outer curve of the aortic arch and thus protect all extracranial vessels. It uses 9 Fr femoral access.
BENEFITS AND CONTROVERSIES
The safety and feasibility of the various EPD are established. EPD may reduce the total volume of brain lesions but a clinically detectable impact remains unsettled.
RELEVANT STUDY ENDPOINTS
Ideally, clinical trials evaluating EPD would be powered to detect differences in major neurological endpoints. This scenario is unlikely given the low disabling stroke rate with TAVI. Because subclinical brain lesions and microinfarcts may be meaningful and correlate with early or late neurocognitive deficit, MRI-based single and total lesion volume seem relevant alternatives. Neurocognitive testing may reveal subtle changes in neurocognitive function at an early stage and may also prove valuable.
PATIENT SELECTION
Cerebral embolisation seems to occur in almost all patients undergoing TAVI for degenerative AS. Therefore, every patient undergoing TAVI could be eligible for EPD provided the respective anatomical requirements are fulfilled. Further research is necessary.
Conflict of interest statement
The Erasmus MC has received research grants from Claret Medical Inc., Medtronic, St. Jude Medical and Abbott Vascular. A. Baumbach has received research grants and consultancy fees from Keystone, St. Jude Medical, Abbott Vascular and Boston Scientific. D. Himbert is a consultant and proctor for Edwards Lifesciences. N. Van Mieghem has received research grants from Claret Medical Inc., Medtronic, St. Jude Medical, Boston Scientific and Abbott Vascular. A. Lansky has received research grants from Keystone. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Deployment of the Embrella device.
Moving image 2. Deployment of the TriGuard device.
Moving image 3. Deployment of the Sentinel device.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Deployment of the Embrella device.
Moving image 2. Deployment of the TriGuard device.
Moving image 3. Deployment of the Sentinel device.

