New cerebral lesions detected with magnetic resonance imaging (MRI) are reported in 67-100% of patients after transcatheter aortic valve implantation (TAVI)1. Large population-based studies associate such MRI lesions with cognitive decline, stroke, and mortality2. The embolisation of debris originating from the aortic root and arch are considered responsible for most periprocedural lesions; hence, cerebral embolic protection (CEP) devices were developed to capture/deflect debris en route to the cerebral circulation.
The PROTEMBO C Trial was an international, multicentre, single-arm trial evaluating the safety and feasibility of the ProtEmbo (Protembis GmbH) CEP system, compared to historical controls (non-inferiority). The COVID-19 pandemic interrupted the PROTEMBO C Trial, and interim results were reported3. This correspondence reflects the final presentation of the results from the completed study in compliance with the original study protocol.
Patients with symptomatic severe aortic stenosis undergoing transfemoral TAVI were eligible for inclusion. The primary safety endpoint was the incidence of major adverse cardiac and cerebrovascular events (MACCE) at 30 days and was compared to a performance goal (PG) of 25%, derived from historical data. A sample size of 60 provided 85% power to reject the null hypothesis (upper limit of 95% confidence interval [CI] of 30-day MACCE with ProtEmbo
The primary performance endpoint was composite technical success defined as 1) successful delivery, deployment, and removal of the device; 2) stable device position; and 3) coverage of the three aortic arch branches. The performance endpoint was site-reported and was compared to a historical PG of 75%. A sample size of 42 patients provided 85% power to reject the null hypothesis (lower limit of the 95% CI with ProtEmbo >PG), assuming a success rate for ProtEmbo of 89% and a 1-sided alpha of 0.02529. Study success required both primary endpoints to be met.
A key secondary efficacy endpoint included the volume of new cerebral lesions assessed by diffusion-weighted magnetic resonance imaging (DW-MRI). DW-MRI brain scans were acquired at baseline and 2-7 days after TAVI and were analysed by an independent core laboratory (Buffalo Neuroimaging Analysis Centre, Buffalo, NY, USA).
Among 64 enrolled patients, 54.7% were female, and the mean age and Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score were 79.5±5.1 years and 2.8±1.3%, respectively. In the intention-to-treat cohort, two patients did not receive the device due to anatomical/access issues. All patients underwent successful TAVI with balloon-expandable (77%) or self-expanding (23%) devices. The incidence of MACCE in the enrolled population at 30 days was 4.7% (3/64), meeting the predefined PG for the primary safety endpoint (upper limit of 95% CI: 12.9% vs PG 25%; p=0.0001) (Figure 1). A single thalamic infarct was reported 12 hours post-procedure in the only patient in which the ProtEmbo device was removed prior to TAVI placement. No device-related adverse events occurred. One single major vascular access site-related complication occurred in a patient requiring a peripheral balloon inflation to stop a bleeding event. Technical success was achieved in 95% of patients in whom the procedure was attempted (intention-to-treat population; 57/60), achieving the predefined PG for the primary performance endpoint (lower limit of 95% CI: 86.3% vs PG 75.0%; p=0.0002) (Figure 1). Complete cerebral coverage was reported in 98.2% of patients, and significant interaction between the TAVI procedure and the ProtEmbo device occurred in one case. The average time for device deployment was 4.7±4.4 minutes, and 84% (47/56) of patients did not receive additional contrast for delivery of the ProtEmbo. The additional fluoroscopy time attributed to ProtEmbo deployment was 4.8±4.1 minutes.
Among the 51 patients undergoing TAVI with successful ProtEmbo use and with both baseline and follow-up DW-MRI (per protocol population), the median total new lesion volume was 210 mm3 (108, 566). As a point of reference, in the SENTINEL trial4, the median total new lesion volume for patients in the no device arm was 310 mm3; the largest lesion volume in a single patient was 681 mm3. All lesions in the remaining patients were <500 mm3 in size, while 76.5% of patients were free of single lesions >150 mm³, and 94.1% were free of single lesions >350 mm³. The distribution of lesions across the regions of the brain is detailed in Table 1.
Overall, in the PROTEMBO C Trial, the ProtEmbo system met its predefined safety and performance endpoints, and encouraging DW-MRI data were observed. The preliminary findings of the trial were confirmed after the entire, originally planned, study cohort was included3.
While the recently published PROTECTED TAVR trial with the SENTINEL (Boston Scientific) CEP system did not reduce the incidence of periprocedural stroke compared to the control group (2.3% vs 2.9%; difference −0.6%; 95% CI −1.7 to 0.5; p=0.30)6, this may be due to the design limitations of the SENTINEL device, as well as low event rates in the trial. The complete DW-MRI dataset suggests that performing TAVI with the ProtEmbo device may be associated with a lower risk of new brain lesions, and of large lesions in particular, than TAVI without CEP56.
In conclusion, the full data from the PROTEMBO C Trial demonstrate that CEP with the ProtEmbo system is safe and feasible. A large, prospective, randomised study evaluating the efficacy of the device is in development (ClinicalTrials.gov: NCT04618718).
Despite advancements in TAVI devices and implantation techniques, embolic stroke remains the most feared and frequent ischaemic procedural complication. It is also hypothesised that silent brain infarcts are associated with significant morbidity and mortality. The ProtEmbo is a novel, low-profile CEP filter delivered via the left radial artery which potentially provides protection for all three cerebral branches of the aorta. The demonstration of the safety and technical feasibility of this device by the PROTEMBO C Trial provides sufficient evidence to proceed with a larger clinical investigation evaluating the efficacy of DW-MRI lesion reduction with the ProtEmbo device.
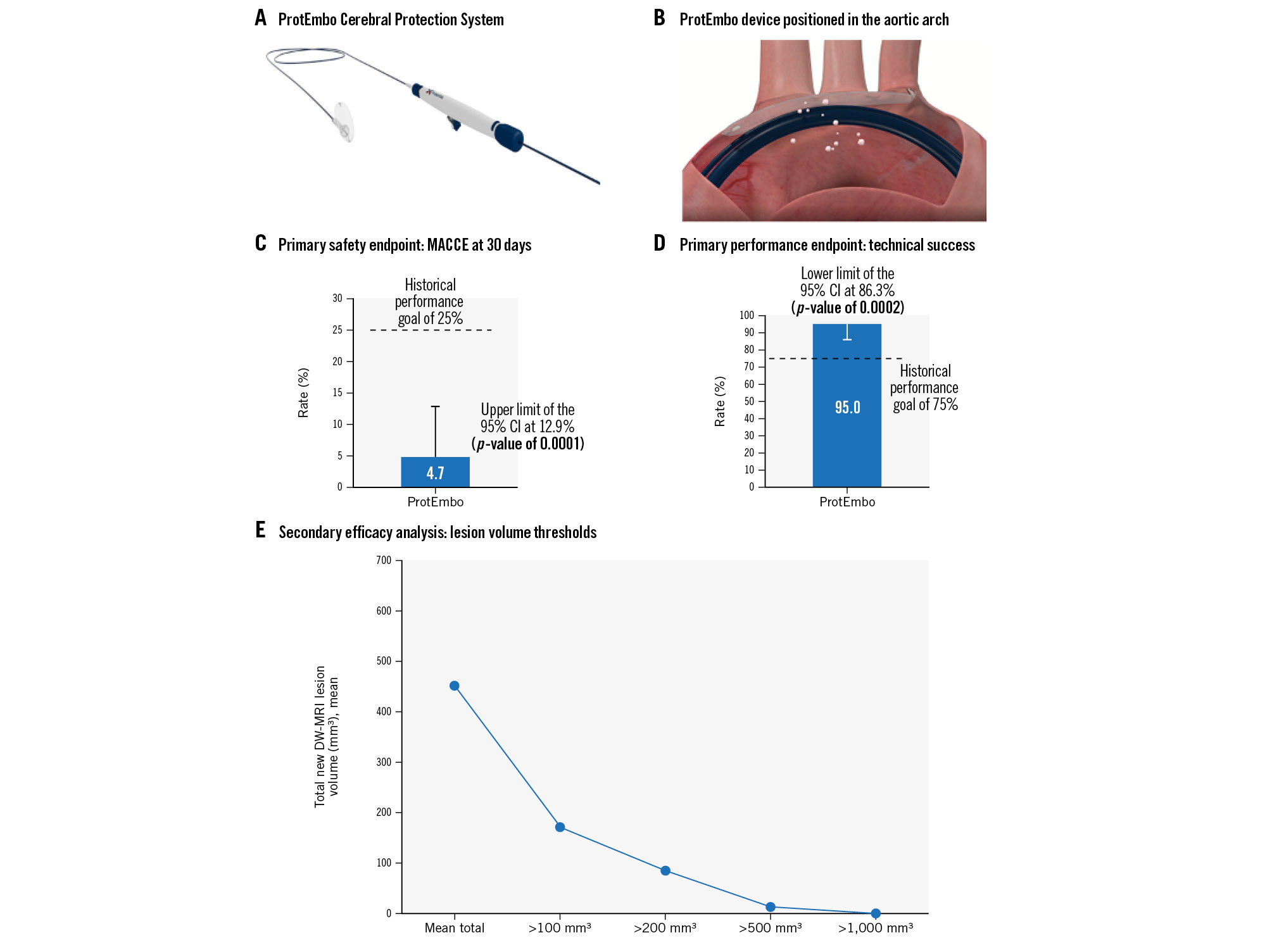
Figure 1. PROTEMBO C Trial: a prospective evaluation of a novel cerebral protection device during TAVI. A) Deployed via a 6 Fr radial guiding sheath from the left radial artery, the ProtEmbo Cerebral Protection System consists of three components: the ProtEmbo device, the ProtEmbo shaper, and the ProtEmbo handle. B) The ProtEmbo is designed to cover the origins of the three vessels supplying blood to the brain and deflect embolic particles away from the cerebral vasculature during TAVI procedures. C) The PROTEMBO C Trial met the primary safety outcome (incidence of major adverse cardiac and cerebrovascular events [MACCE] at 30 days) and D) primary performance outcomes (successful delivery, deployment, and removal of the device, stable device position and coverage of the three aortic arch branches), compared with historical performance goals. The secondary efficacy analysis of new lesion volume on DW-MRI brain scans in the per-protocol analysis (E) showed the median total new lesion volume was 210 mm3 (108, 566), with only a single lesion above 500 mm3 in size, 76.5% of patients free of single lesions >150 mm³, and 94.1% free of single lesions >350 mm³. CI: confidence interval; DW-MRI: diffusion-weighted magnetic resonance imaging; TAVI: transcatheter aortic valve implantation
Table 1. Distribution of new lesions on DW-MRI brain scans.
| Right | Brain stem | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cerebral | Putamen | Occipital | Parietal | Cerebellum | Frontal | Caudate | Thalamus | Temporal | |
| 56 | 15 | 44 | 54 | 48 | 77 | 48 | 54 | 36 | 48 |
| Left | |||||||||
| Cerebral | Putamen | Occipital | Parietal | Cerebellum | Frontal | Caudate | Thalamus | Temporal | |
| 51 | 111 | 45 | 42 | 48 | 51 | 60 | 39 | 42 | |
| Total new lesion volume, median (mm3) in different areas of the brain. DW-MRI: diffusion-weighted magnetic resonance imaging | |||||||||
Acknowledgements
The authors wish to acknowledge the members of the independent data and safety monitoring board (Darren Mylotte, MD; Dana Leifer, MD; Sameer Gafoor, MD; and Isaac George, MD) for their contributions to the PROTEMBO C Trial, and Victor Jimenez, MD, for proctoring the first-time use of the investigational device.
Funding
This clinical trial was supported by an unrestricted research grant from Protembis GmbH.
Conflict of interest statement
D. Jagielak has received proctoring and lecture fees from Meril Life Sciences. M. Abdel-Wahab’s institution has received consultancy fees and/or speaker honoraria on his behalf from Abbott, Medtronic, and Boston Scientific. N. Werner has received proctoring and lecture fees from Edwards Lifesciences and Medtronic. A.R. Witkowski has received proctoring and lecture fees from Edwards Lifesciences and Medtronic. M. Grygier has received proctoring and lecture fees from Boston Scientific, Medtronic, Abbott, and Edwards Lifesciences. M. Adam has received personal fees from Edwards Lifesciences and Boston Scientific; has received grants and personal fees from Medtronic; and has been a proctor for Medtronic. F. Gatto has received proctoring fees from Boston Scientific and Medtronic. T. Schmidt has received travel expenses from Protembis. D. Mylotte has been a consultant for Boston Scientific, Medtronic, and MicroPort. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.

