
Introduction
A substantial proportion of patients submitted to transcatheter aortic valve replacement (TAVR) continues to be affected by procedure-related neurological events, with 30-day clinical stroke rates in the range of 4-7%. Also, routine neuroimaging studies reveal that ischaemic cerebral infarction caused by showers of cerebral emboli during valve instrumentation and placement affect virtually all patients undergoing TAVR1,2,3.
In order to prevent these embolic phenomena, several cerebral embolic protection devices (CEPD) have recently been developed. Their use during TAVR has been associated with improved early imaging and clinical neurological outcomes1.
The TriGuard™ and TriGuard™ HDH (Keystone Heart, Tampa, FL, USA) have previously shown reductions in new ischaemic brain lesions and total lesion volume (TLV) per patient in diffusion-weighted magnetic resonance imaging (DW-MRI) in the DEFLECT I and III trials2,3.
The present study was a prospective, single-centre, single-arm pilot study to evaluate the safety and performance of the TriGUARD™ 3 CEPD in patients undergoing TAVR.
Methods
Patients were selected if they were eligible for transfemoral TAVR and had no contraindications to cerebral MRI.
The primary performance endpoint was device performance, defined as successful device deployment at the aortic arch, positioning with complete three-vessel coverage throughout the procedure and retrieval without interference with the TAVR procedure. The primary safety endpoint was in-hospital device-related safety, a hierarchical composite of cardiovascular death, ischaemic stroke, life-threatening or disabling bleeding and acute kidney injury.
Important secondary safety and imaging efficacy endpoints were also pre-specified. Details are available in Supplementary Appendix 1.
THE TriGUARD 3 DEVICE (Figure 1)
The TriGUARD 3 CEPD is a biocompatible single-sized deflection device composed of a structural radiopaque nitinol frame and an ultra-thin polymer mesh (nominal pore size 115×145 μm) that allows maximal blood flow to the brain while diverting emboli towards the descending aorta (Figure 1C). The device is heparin coated to reduce thrombogenicity and increase lubricity. The full system also includes a delivery subsystem for crimping and loading the device into an 8 Fr sheath introduced transfemorally. Under fluoroscopic guidance, after performing an aortic arch angiogram, the device is positioned in the aortic arch to cover all three major cerebral arteries (Figure 1A, Figure 1B).
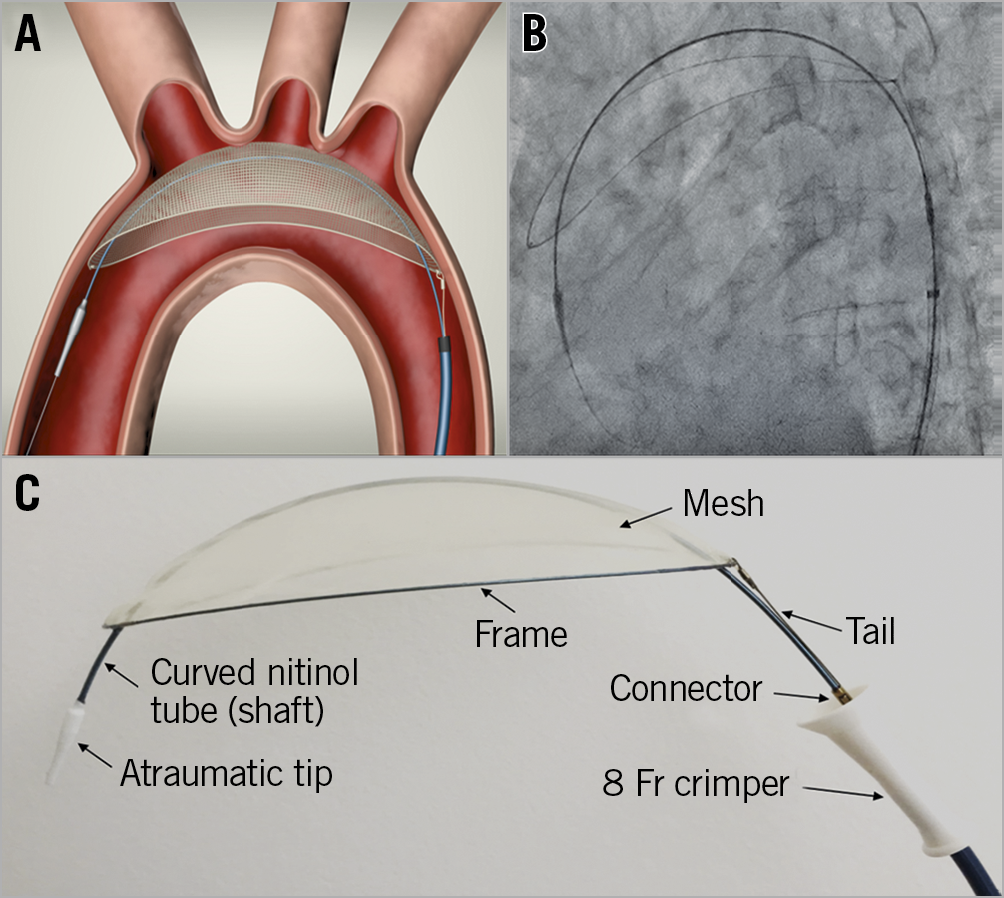
Figure 1. The TriGUARD 3 after deployment in the aortic arch covering the three major cerebral vessels. A) Schematic representation. B) Under fluoroscopy. C) Device details.
Results
A pre-specified total of 10 consecutive subjects were enrolled in the study between November and December 2018.
Baseline patient and procedural characteristics are outlined in Supplementary Table 1 and Supplementary Table 2.
The primary device performance endpoint was met in 90% of subjects (Table 1). The TriGUARD 3 device was successfully deployed with correct orientation on the first attempt in all patients (100%). The positioning with complete three-vessel coverage throughout the TAVR procedure was reported in 90% of subjects (partial coverage was reported in one patient after core lab analysis). The device was successfully retrieved in all cases (100%) and there was no device interference with the TAVR procedure.
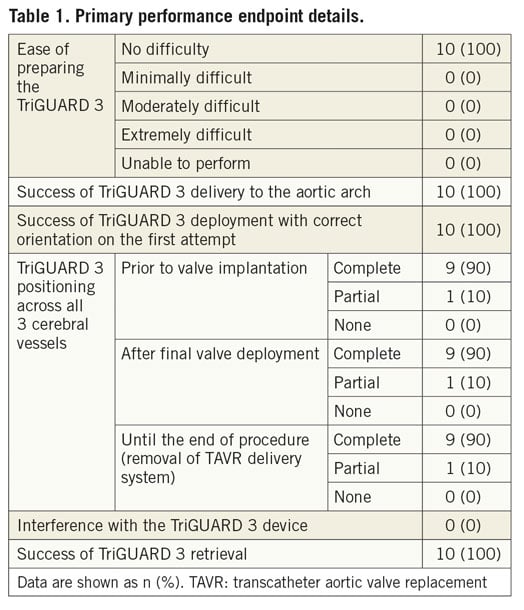
The primary safety endpoint of in-hospital device-related safety did not occur in the study population (0%).
A single in-hospital major adverse cardiovascular and cerebral event (MACCE) occurred (10%), consisting of one vascular complication (haematoma) in a patient who also had a concurrent major bleeding event (not a MACCE event). Both events were related to the TriGUARD 3 vascular access site and considered procedure-related.
The cerebral DW-MRI demonstrated new ischaemic lesions in all available patients with a median TLV of 200.23 mm 3. Details of the secondary imaging efficacy endpoints are shown in Supplementary Table 3.
Discussion
The present study demonstrated an overall efficacy performance of the TriGUARD 3 of 90% with a single case of partial coverage of the cerebral vessels. Although this was not apparent during the procedure, careful core lab analysis concluded that the device was placed too proximal into the ascending aorta with partial coverage of the left vertebrobasilar artery. Due to design iterations, the TriGUARD 3 maintains a stable and correct position in the aortic arch throughout the entire TAVR procedure, without displacement or any interference with the TAVR delivery system, and thus provides improved performance compared to previous generations (performance success rate of 80% for the TriGuard in the DEFLECT I study and 89% for the TriGuard HDH device in the DEFLECT III trial)2,3.
Importantly, in this study, the use of the TriGUARD 3 device was safe with only one (10%) single vascular complication (device access-related groin haematoma) without any other MACCE. These early safety results are also better than the ones from the previous device versions (16.2% overall in-hospital MACCE and 5.4% stroke in the DEFLECT I study and 21.7% overall in-hospital MACCE and 4.3% stroke in the DEFLECT III trial)2,3.
The cerebral DW-MRI demonstrated new ischaemic lesions in all patients, without neurological symptoms, confirming the importance of cerebral embolisation prevention. Although speculative, the reasons for these MRI findings might be based on the fact that the pore size of the mesh (115×145 μm) still allows small particles to pass and secondly be the result of debris dislodgement shortly after the TAVR procedure. Due to different trial methodologies, changes in MRI acquisition parameters and even evolution of the TAVR procedure over time, it is not possible to establish true comparisons with the previous TriGuard versions or other devices; however, the TLV seems to be reduced compared to previous TriGuard versions or other devices.
Limitations
Given the small sample size, no hypothesis testing was performed and no conclusive comparison with unprotected TAVR, the prior-generation TriGuard devices or other CEPD can be made.
Conclusion
This pilot study shows that the use of the TriGUARD 3 for cerebral protection during TAVR is feasible and safe. The device was successfully delivered, deployed, and retrieved without interference with the TAVR procedure in 100% of cases, and achieved complete three-vessel cerebral embolic protection throughout the procedure in 90% of cases.
|
Impact on daily practice The TriGUARD 3 enhances and facilitates periprocedural cerebral protection which might represent a step forward regarding this important matter during TAVR. |
Funding
This study was funded by Keystone Heart.
Conflict of interest statement
M.P. Margolis is Chief Medical Officer at Keystone Heart. A. Lansky and P. Stella have served on the advisory board for Keystone Heart in the past. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

