Abstract
Background: Stroke and other clinically significant embolic complications are well documented in the early period following transcatheter aortic valve replacement (TAVR). The CAPTIS device is an embolic protection system, designed to provide neurovascular and systemic protection by deflecting debris away from the brain’s circulation, capturing the debris and thus avoiding systemic embolisation.
Aims: We aimed to study the safety and feasibility study of the CAPTIS complete cerebral and full-body embolic protection system during TAVR.
Methods: A first-in-human study investigated the safety, feasibility and debris capturing ability of CAPTIS during TAVR. Patients were followed for 30 days. The primary endpoints were device safety and cerebrovascular events at 72 hours.
Results: Twenty patients underwent TAVR using balloon-expandable or self-expanding valve systems. CAPTIS was successfully delivered, positioned, deployed, and retrieved in all cases, and TAVR was successfully completed without device-related complications. No cerebrovascular events were observed. High numbers of debris particles were captured in all patients.
Conclusions: The use of the CAPTIS full-body embolic protection system during TAVR was safe, and it captured a substantial number of debris particles. No patient suffered from a cerebrovascular event. A randomised clinical trial is warranted to prove its efficacy.
Introduction
Transcatheter aortic valve replacement (TAVR) has transformed the treatment of aortic stenosis (AS). Technological progress and increased operator experience have significantly improved the procedural safety and efficacy. Yet, periprocedural stroke occurs in 2-5% of TAVR cases123 and is associated with increased mortality and significant morbidity3.
Embolic protection devices (EPD) were developed to mitigate the risk of emboli and neurological complications. Several studies have reported conflicting results regarding the association of first-generation EPD with patient outcomes4567. The major limitations of the currently available EPD are their partial coverage of the great vessels supplying the brain and the instability of the deflection device inside the aortic arch during TAVR.
The CAPTIS (Filterlex) system is designed to provide neurovascular (brain) and systemic protection by deflecting debris away from the brain’s circulation, capturing the debris and avoiding systemic embolisation. The system is composed of a filter-deflector that covers all the great vessels supplying the brain. The deflector is stabilised by an aortic anchoring structure containing filters that capture embolised particles
We report herein the feasibility and safety results of the first-in-human study of the CAPTIS EPD during TAVR (ClinicalTrials.gov: NCT04659538).
Methods
The study was a prospective first-in-human evaluation of CAPTIS feasibility and safety during TAVR for severe AS. Inclusion criteria included clinical indication for AS treatment, anatomical suitability for transfemoral TAVR and a femoral access site diameter ≥6 mm. To accommodate the CAPTIS device, the descending aorta − measured 10 cm distal to the left subclavian artery − had to have a diameter of 20-27 mm. The distance between the innominate artery and left subclavian arteries (including lumens) had to be <65 mm. The exclusion criteria are detailed in Supplementary Appendix 1.
All patients signed the informed consent. The study protocol was approved by the ethical and safety committee of the Israeli Ministry of Health and by the local institutional review boards of the individual sites. The study was sponsored by Filterlex Medical Ltd (Caesarea, Israel).
STUDY DEVICE
The CAPTIS device (Central illustration) is a single-use, temporary, repositionable and retrievable embolic protection system consisting of three parts: 1) a self-expanding deflecting filter composed of a nitinol frame and a polymer mesh filter made of polyether ether ketone (145x115 μm pore size, 58 μm thick) designed to cover all three great vessels that supply the brain; 2) a rail connecting and stabilising the deflecting filter to the anchoring structure; 3) a self-expanding anchoring nitinol structure that is available to investigators in two sizes: S – for a descending aorta inner diameter of 20 to 23 mm − and M – for a diameter of 23 to 27 mm. The anchor contains two filter pockets made of the same material as the deflector.
CAPTIS is inserted through the same TAVR femoral access site that allows delivery of the TAVR systems. A thin cable is secured to the anchor tip to ensure continuous device grip.
Following valve deployment, the CAPTIS device is retrieved using a dedicated retrieval catheter.
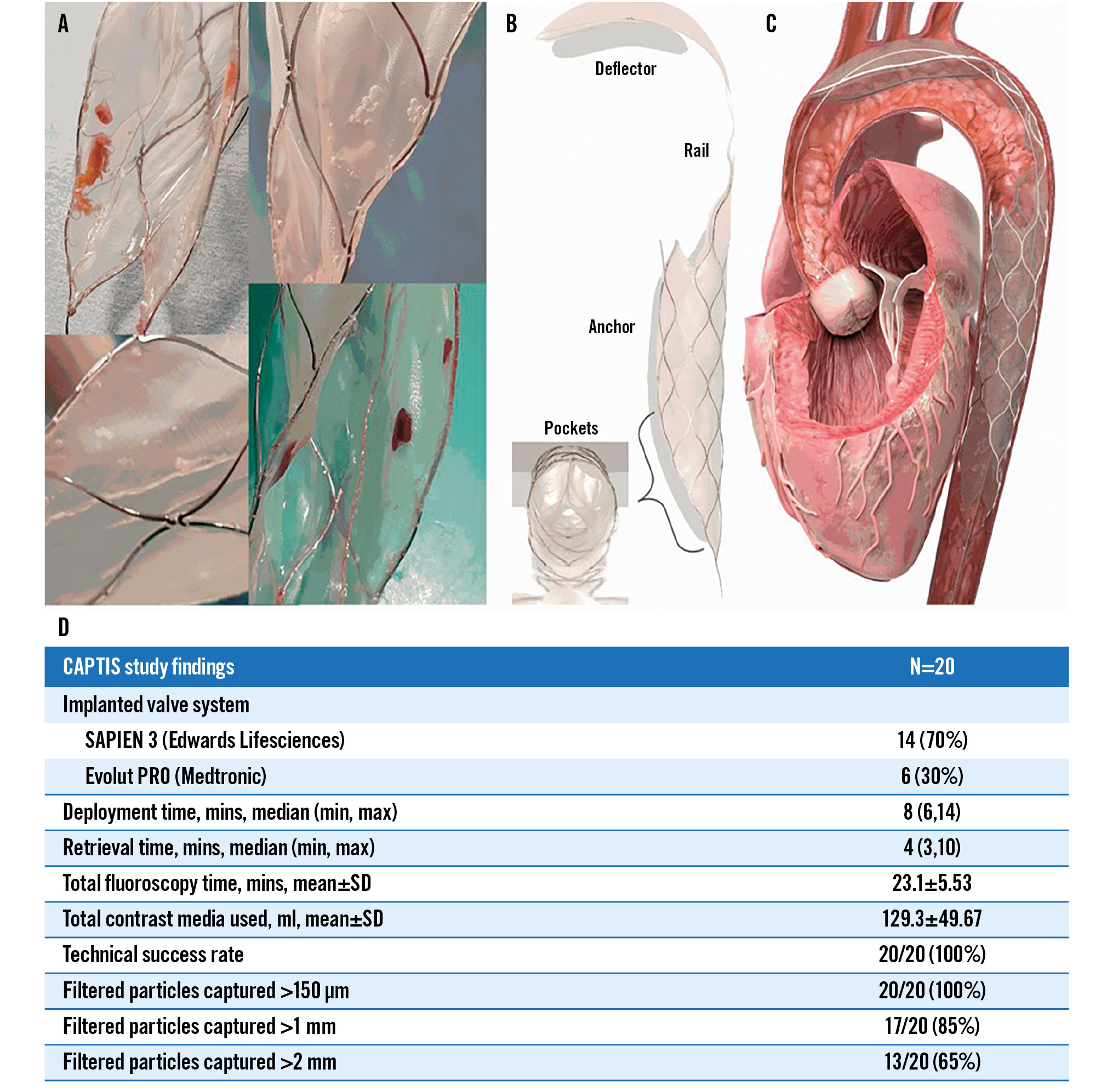
Central illustration. CAPTIS embolic protection device design and major first-in-human study findings. A) Examples of large debris collected with the CAPTIS system. B) The CAPTIS device with its three main components: deflector, rail and anchoring structure. Filtering pockets are located within the stent. C) The CAPTIS device is positioned with the deflector filter covering all the vessels that provide blood flow to the brain. The filtering pockets protect the lower part of the body, and the debris is removed from the body at the end of the procedure. D) The chart features the key characteristics and findings of the CAPTIS first-in-human trial. SD: standard deviation
STUDY PROCEDURE
The pre-TAVR chest, abdomen and pelvis computed tomography angiography was analysed by an independent core laboratory (Cedars-Sinai, Los Angeles, CA, USA). Patients were approved by an eligibility committee.
TAVR was performed according to institutional standards. CAPTIS was inserted via a 16 Fr sheath and deployed under fluoroscopic guidance with the deflector covering the ostia of the innominate artery, left carotid and left subclavian arteries. The anchoring structure was deployed at the upper descending aorta. The valve system was delivered to the aortic annulus inside the anchoring structure in between the filter pockets. Following TAVR, CAPTIS was fully retrieved, and the filters were sent for histopathological analysis.
Patients underwent a comprehensive neurological assessment by an independent neurologist with evaluation of the National Institutes of Health Stroke Scale (NIHSS) score prior to TAVR, and at 72-hour and 30-day follow-ups.
STUDY ENDPOINTS
Primary safety endpoints were 1) occurrence of all major adverse and cerebrovascular events (MACCE) at 72 hours post-procedure − MACCE was defined as all-cause death and all cerebrovascular events; and 2) device-related complications at 72 hours.
Secondary safety endpoints were 1) all MACCE at 30 days post-procedure; 2) the number of stroke events at 30 days; 3) the number of transient ischaemic stroke (TIA) events at 30 days; and 4) acute kidney injury (defined as an increase from baseline in creatinine levels of 25% or 0.5mg/dL at 72 hours [or discharge]).
Secondary feasibility and technical performance endpoints were 1) capture and removal of debris (with histopathological examination); and 2) technical device performance, defined as the successful implantation of the TAVR device (including delivery, positioning and deployment) and complete retrieval of the CAPTIS device. Complications were reported according to the Valve Academic Research Consortium (VARC)-3 definitions8.
HISTOPATHOLOGICAL ANALYSIS
Devices were stored in formalin and analysed in a histopathology core lab (CVPath Institute, Gaithersburg, MD, USA).
STATISTICAL ANALYSIS
Appropriate descriptive statistics were computed for all safety, feasibility, and technical performance parameters.
Binary variables are summarised using frequencies and percentages. Continuous variables are presented as mean and standard deviation (SD) and discrete variables as median with interquartile range (IQR 25-75).
Results
Twenty patients were enrolled at two medical centres. Patient baseline characteristics are detailed in Table 1. TAVR procedural data are detailed in Table 2. Fourteen of the implanted valves (70%) were balloon-expandable SAPIEN 3 (Edwards Lifesciences) valves and six (30%) were self-expanding Evolut PRO (Medtronic) valves. The median CAPTIS deployment time was 7 minutes, and the median retrieval time was 4 minutes. Technical success of the CAPTIS device and successful TAVR were achieved in all patients.
There were no mortalities, neurological events or changes in the NIHSS score at 72 hours or 30 days. Mild, reversible creatinine elevation was noted in one patient. Two patients (10%) had access site-related bleeding (one from the contralateral groin) which required blood transfusion (Bleeding Academic Research Consortium [BARC]-3a)8.
Histopathological analysis of the filters yielded particles in all twenty cases. The average number of particles per patient was 1,448, with an average of 112 particles >150 μm. Particles >1 mm were detected in 17 patients (85%), and particles >2 mm were detected in 13 (65%). Particles made of fresh and organised thrombus, valve tissue and arterial wall tissue were observed in all filters. Calcium particles were identified in 9 (45%) filters, foreign material in 18 (90%) and myocardial tissue in 4 (20%). Supplementary Table 1 details the number and size of particles per patient.
Table 1. Demographic and baseline characteristics.
| Total (N=20) | |
|---|---|
| Age, years | 76.6±5.0 |
| Female | 9 (45) |
| BMI, kg/m2 | 29.9±4.5 |
| Diabetes mellitus | 14 (70) |
| Dyslipidaemia | 17 (85) |
| Hypertension | 17 (85) |
| Prior CABG | 4 (20) |
| Prior PCI | 8 (40) |
| Values are mean±SD or n (% of total cohort). BMI: body mass index; CABG: coronary artery bypass graft; PCI: percutaneous coronary intervention; SD: standard deviation | |
Table 2. Procedural characteristics.
| N=20 | |
|---|---|
| Implanted valve system | |
| SAPIEN 3 (Edwards Lifesciences) | 14 (70) |
| Evolut PRO (Medtronic) | 6 (30) |
| Deployment time, mins | 8 (6,14) |
| Retrieval time, mins | 4 (3,10) |
| Predilation | 7 (35) |
| Post-dilation | 0 (0) |
| Total fluoroscopy time, mins | 23.1±5.53 |
| Total contrast media used during procedure, ml | 129.3±49.67 |
| Values are median (min, max), n (% of total cohort) or mean±SD. | |
Discussion
The CAPTIS device is designed to cover and deflect particles away from all three epiaortic arteries and provide full brain protection from debris embolisation during TAVR. Furthermore, CAPTIS captures embolic debris, thus, providing systemic protection as well. The CAPTIS system was safely deployed before TAVR and safely retrieved after TAVR with minimal interaction with the TAVR procedure. There were no device-related complications.
Postprocedural stroke remains a dreaded complication of TAVR, with a high morbidity and mortality burden, as an estimated 2-5% of patients suffer peri-TAVR stroke at various levels of clinical severity123. Furthermore, a majority of TAVR patients have new brain lesions detected by diffusion-weighted magnetic resonance imaging (MRI)4, which may have future neurological consequences.
The optimal embolic protection device should provide full brain protection throughout the TAVR procedure. It should be easy to deliver and retrieve, be stable in the aortic arch, and not affected by the relatively stiff and bulky TAVR system. Furthermore, it should not require an additional or a larger access route. In our first-in-human study, CAPTIS appears promising in fulfilling these requirements. CAPTIS deployment and retrieval were quick and successful in all cases. The deflector was stable, covered the ostia of the cerebrovascular arteries and deflected particles to the filtering pockets. CAPTIS was deployed via the arteriotomy in the femoral artery that was also used for the introduction of the TAVR system – with no need for an additional access site or arteriotomy upsizing. This differs from other EPDs: SENTINEL (Boston Scientific) is delivered through a dedicated right radial arteriotomy, TriGUARD 3 (Keystone Heart) requires a 9 Fr sheath in the contralateral leg, and other early-stage protection devices require either radial (right or left) or femoral contralateral arterial access.
CAPTIS provides embolic protection not only to the brain. The filter pockets inside the anchor collect deflected debris that are removed from the body upon procedural completion. Systemic emboli were reported following TAVR in other studies, causing renal, mesenteric, and lower limb ischaemia910. Moreover, embolisation is one of the mechanisms of acute kidney injury post-TAVR, occurring in 10-20% of patients, and is associated with increased morbidity and mortality11. Thus, capturing deflected particles from the aortic arch protects the lower part of the body.
Histopathological analysis of the captured debris identified very large numbers of particles of varying sizes. Particles larger than 150 μm are regarded as having an increased potential of causing brain ischaemic injury. The number of particles retrieved by CAPTIS were 2-3 times the number of particles reported with the SENTINEL device5. This may result from a larger volume of blood filtered by the CAPTIS device. The histological types of debris were similar to those detected in the SENTINEL filter and included calcium, valve, myocardial, and vascular tissues, as well as thrombus and foreign materials5.
Limitations
Our study has several limitations. The study was not designed to test for efficacy; clinical outcomes cannot be concluded due to the small cohort, and the lack of cerebral MRI. The clues for safety and potential efficacy were found in the meticulous pre- and postprocedural neurological assessment of the captured debris. The device was available in limited sizes, and patients with a descending aorta diameter smaller than 20 mm or larger than 27 mm were excluded as well as patients with challenging vascular features. Thus, these study findings should be considered in view of the study objectives: proving the feasibility and safety of CAPTIS during TAVR and providing an encouraging signal for a larger controlled trial in a larger and higher-risk cohort.
Conclusions
CAPTIS is a novel full-body embolic protection device designed to reduce neurological and systemic embolic complications during TAVR. CAPTIS use is streamlined with current practice, without the need for additional or upsized arteriotomies or exceptional manoeuvres. This first-in-human study shows that CAPTIS deployment and retrieval are feasible and safe. No neurological adverse events were observed. Histopathological evaluation of captured debris yielded large numbers of particles of various sources and sizes. Further studies are warranted to evaluate the efficacy of CAPTIS in preventing brain injury post-TAVR.
Impact on daily practice
CAPTIS is a device designed to provide full cerebral and systemic embolic protection. The use of the CAPTIS protection device is feasible and safe during TAVR. High numbers of debris particles were captured in all patients in this study. CAPTIS is introduced via the same access as the TAVR systems, not calling for an additional arteriotomy and it is compatible with all commercial TAVR systems. Randomised clinical trials are now warranted to prove the efficacy of CAPTIS.
Conflict of interest statement
H. Danenberg serves as a clinical proctor for Edwards Lifesciences and Medtronic. R. Makkar serves on the medical advisory board of Filterlex Medical Ltd. G. Weisz is co-founder and medical director of Filterlex Medical Ltd. S. Eli is co-founder and CEO of Filterlex Medical Ltd. E. Teichman is co-founder and CTO of Filterlex Medical Ltd. Y. Mezape isan employee of Filterlex Medical Ltd. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

