Abstract
Periprocedural stroke after transcatheter aortic valve implantation (TAVI) remains a significant issue, which is associated with high morbidity, and is increasingly important as intervention shifts to younger and lower-risk populations. Over the last decade of clinical experience with TAVI, the incidence of periprocedural stroke has stayed largely unchanged, although it is prone to underreporting due to variation in ascertainment methods. The aetiology of stroke in TAVI patients is multifactorial, and changing risk profiles, differing study populations, and frequent device iterations have made it difficult to discern consistent risk factors. The objective of this review is to analyse and clarify the contemporary published literature on the epidemiology and mechanisms of neurological events in TAVI patients and evaluate potential preventive measures. This summary aims to improve patient risk assessment and refine case selection for cerebral embolic protection devices, while also providing a foundation for designing future trials focused on stroke prevention.
Many patients undergoing transcatheter aortic valve implantation (TAVI) consider stroke to be a complication worse than death. Despite significant advancements in valve technology and refinements in procedural techniques over the last decade, the rates of periprocedural stroke (occurring within 30 days of the procedure) appear remarkably unchanged1. Further, though real-world, self-reported registry data report consistently low rates of periprocedural stroke (~2.3%), these rates are discrepant from prospective clinical trial data over the same time period (0.6% to 6.7%); this is likely due to the more stringent protocols for stroke ascertainment that are used in clinical trials, which can include routine systematic examination by a neurologist or use of brain magnetic resonance imaging (MRI). Reported stroke rates have also been shown to be higher in comprehensive stroke centres (CSCs) compared to non-CSCs, even after adjusting for elevated baseline risk, which is commonly seen in patients in tertiary referral centres2. Finally, silent brain infarction (SBI), or covert stroke, is underrecognised after TAVI but can have downstream deleterious effects on neurocognition.
In this article, we provide an overview of the contemporary epidemiology of TAVI-associated stroke, as well as the mechanisms and risk factors for ischaemic events. Strategies for periprocedural stroke prevention are evolving; we herein review the current evidence and indications for postprocedural monitoring and preventative antithrombotic therapies and summarise the landscape of cerebral embolic protection (CEP) devices and future trial design considerations.
Epidemiology of ischaemic stroke in TAVI
When evaluating the incidence of stroke after TAVI, it is important to note that stroke, unlike other quality endpoints for valve intervention, is uniquely dependent on the conviction of ascertainment. Studies with scheduled neurological assessment or diffusion-weighted imaging (DWI; i.e., trials of CEP devices) have reported higher stroke rates compared to self-reported registry and observational data (Figure 1)3. Similarly, CSCs, with the infrastructure for prompt detection of symptoms and diagnostic imaging, have higher reported rates of stroke compared to non-CSCs2. Nonetheless, trial and observational data consistently indicate a clear cause-and-effect relationship between TAVI and stroke during the periprocedural period (within 30 days) as well as the early phase (between 30 days and 1 year)4. It is important to note that the occurrence of late stroke (beyond 1 year) following TAVI seems to align with the projected rate based on pre-existing comorbidities and age, without any additional impact from the TAVI procedure itself4.
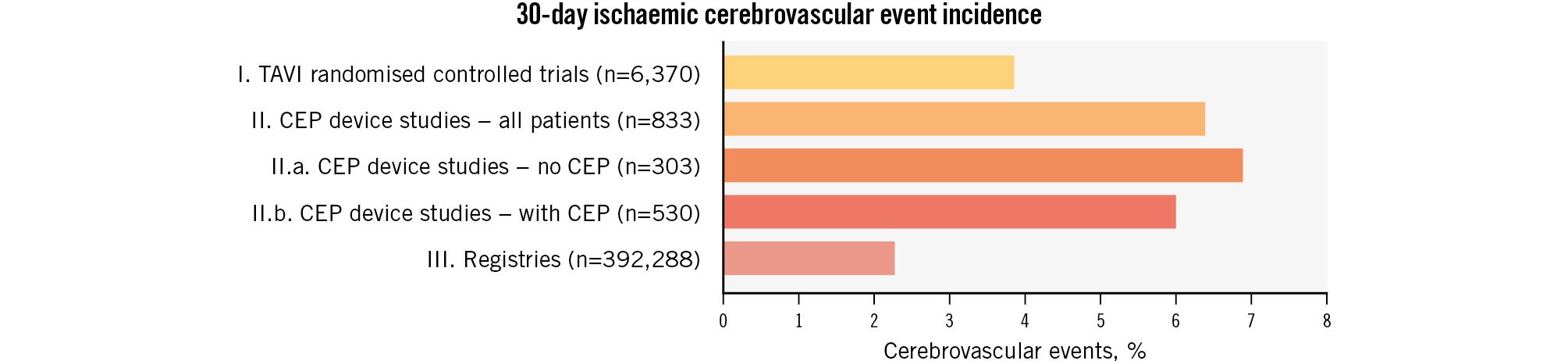
Figure 1. Stroke rate after TAVI according to study design. Printed with permission from Springer Link3. CEP: cerebral embolic protection; TAVI: transcatheter aortic valve implantation
PERIPROCEDURAL ISCHAEMIC STROKE
The rate of stroke after TAVI correlates most reliably with the overall surgical risk profile of a patient (Table 1). The PARTNER 1A trial, conducted in high-risk patients, showed a 30-day stroke rate of 6.7%5, while more recent results from the PARTNER 3 trial, in low-risk patients, demonstrated a 0.6% risk of periprocedural stroke6. However, despite the expansion of TAVI into lower-risk populations, the overall periprocedural stroke rate after TAVI has remained between 2.3% and 2.8% since 2012 (Central illustration). Among high- to extreme-risk patients, the periprocedural stroke rate has not changed (2.8% in 2012, 2.7% in 2019)1.
It is unclear why the periprocedural stroke rate has not changed significantly; the lower overall risk of the TAVI population along with the advances in device technology employing smaller and more deliverable devices would be expected to reduce the stroke rate. It is possible that any reduction in stroke over time has been offset by more widespread TAVI interventions that dilute operator expertise (2012: 198 US sites performing TAVI vs 2020: 701 US sites) or that more complex cases (not represented by Society of Thoracic Surgeons [STS] risk scores) are being undertaken. Indeed, more contemporary TAVI cohorts may include patients at higher risk for stroke, including those undergoing valve-in-valve (ViV) procedures (2011-2013: 2.2% vs 2019: 6.3%) and bicuspid cases (2015: 2.8% of all TAVI vs 2020: 6.8%)17.
Table 1. Stroke rate in randomised controlled trials of TAVI.
| Study | Year | N | Age, years | STS-PROM, % | Non-disabling stroke, % | Disabling stroke, % | Any stroke, % |
|---|---|---|---|---|---|---|---|
| PARTNER cohort B | 2010 | 348 | 83.6 | 11.8 | 0.9 | 3.8 | 4.7 |
| PARTNER cohort A | 2011 | 179 | 83.9 | 11.2 | 1.7 | 5.0 | 6.7 |
| CoreValve High Risk | 2014 | 390 | 83.2 | 7.3 | 1.0 | 3.9 | 4.9 |
| CHOICE (BEV) | 2014 | 121 | 81.9 | 5.6 | NR | NR | 5.8 |
| CHOICE (SEV) | 2014 | 117 | 79.6 | 6.2 | NR | NR | 2.6 |
| NOTION | 2015 | 145 | 79.9 | 2.9 | NR | NR | 1.4 |
| PARTNER 2 | 2016 | 1,011 | 81.5 | 5.8 | 2.3 | 3.2 | 5.5 |
| SURTAVI | 2017 | 864 | 79.9 | 4.4 | 2.2 | 1.2 | 4.5 |
| PORTICO-1† | 2018 | 941 | 82.4 | 5.8 | 1.0 | 1.6 | 2.6 |
| REPRISE I§ | 2018 | 601 | 82.8 | 6.7 | 2.8 | 2.0 | 4.8 |
| PARTNER 3 | 2019 | 496 | 73.3 | 1.9 | 0.6 | 0.0 | 0.6 |
| Evolut Low Risk | 2019 | 725 | 74.1 | 1.9 | 3.0 | 0.5 | 3.4 |
| SCOPE I‡ | 2020 | 372 | 82.6 | 3.7 | 1.0 | 1.0 | 2.0 |
| UK TAVI | 2022 | 458 | 81.0 | 2.6 | NR | NR | 2.4 |
| †Stroke rate for Portico arm displayed; ‡stroke rate for ACURATE neo arm displayed; §stroke rate for LOTUS arm displayed. BEV: balloon-expandable valves; NR: not rated; SEV: self-expanding valves; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI: transcatheter aortic valve implantation | |||||||
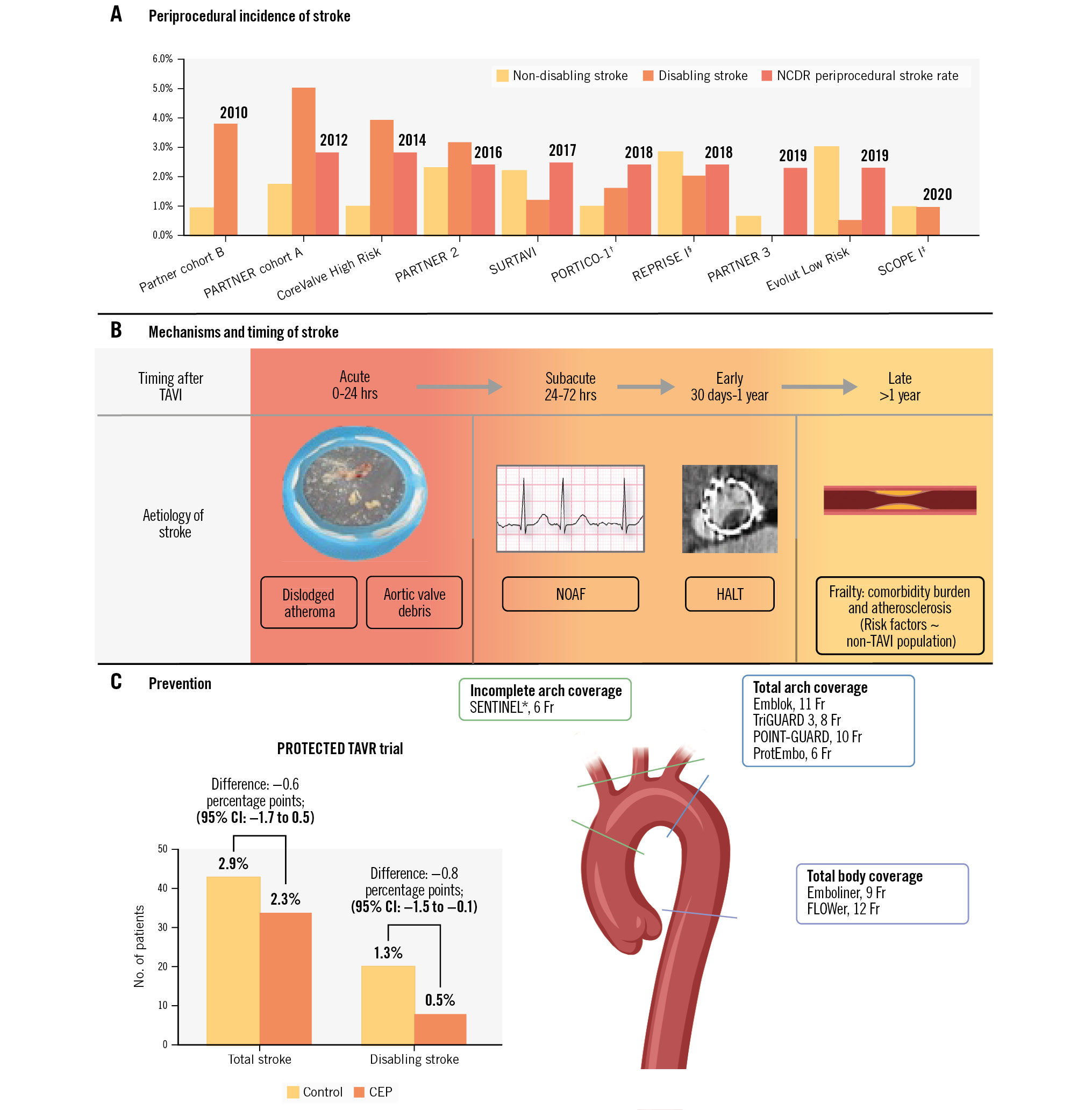
Central illustration. Cerebrovascular events and transcatheter aortic valve implantation. A) Periprocedural incidence of TAVI-related stroke reported in randomised control trials of TAVI and NCDR by year. B) Mechanisms of stroke and their timing relative to TAVI. C) Prevention of stroke after TAVI; PROTECTED TAVR trial results and vessel coverage of approved and experimental cerebral embolic protection devices. §Stroke rate displayed for the LOTUS (Boston Scientific) arm. †Stroke rate displayed for the Portico (Abbott) arm. ‡Stroke rate displayed for the ACURATE neo (Boston Scientific) arm. *U.S. Food and Drug Administration-approved device. Emblok (Innovative Cardiovascular Solutions); Emboliner (Emboline); FLOWer (AorticLab); POINT-GUARD (Transverse Medical); ProtEmbo (Protembis); SENTINEL (Boston Scientific); TriGUARD 3 (Keystone Heart). CEP: cerebral embolic protection; CI: confidence interval; Fr: French; HALT: hypoattenuated leaflet thickening; NCDR: National Cardiovascular Data Registry; NOAF: new-onset atrial fibrillation; TAVI: transcatheter aortic valve implantation
Silent brain infarction and neurocognitive decline
Unrecognised SBI is common after TAVI and may lead to neurocognitive decline. Transcranial Doppler studies performed during TAVI demonstrate debris embolisation in virtually all cases, though not all emboli will induce infarct8. Studies that performed DWI pre- and post-TAVI found SBI in 73.7% of patients9.
Neurocognitive function following TAVI has become an increasingly recognised component of quality of life, and large observational data have indicated an association between SBI after TAVI and the incidence of neurocognitive decline9. However, few trials formally assess neurocognitive decline after TAVI due to inherent difficulties in testing (Table 2).
Table 2. Silent brain injury and neurocognitive decline summary28293031323334.
| Neurocognitive decline after TAVI | Limitations of neurocognitive testing |
|---|---|
| May occur after TAVI due to silent brain infarcts in fronto-subcortical pathways which are subject to vascular injury | Baseline deficits are common among TAVI patients, which can reduce the specificity of testing |
| Deficits often seen in executive function and processing speed | Cognitive domains assessed by each test are variable |
| Multiple neurocognitive tests are available to assess cognitive function | Tests are cumbersome to administer and trained personnel are needed |
| Cognitive decline may not be apparent for 3-5 years | Long-term, serial follow-up is needed to see effects |
| Transient cognitive decline may occur from the anaesthesia or prolonged hospitalisation | Changes in score may be statistically but not clinically significant |
| TAVI: transcatheter aortic valve implantation | |
Mechanisms and risk factors for stroke
The mechanism of stroke generally varies according to the time elapsed from transcatheter heart valve (THV) implantation. Stroke that occurs in the acute period (<24 hours) is more likely related to embolisation of aortic valve debris or thrombus that forms during the procedure or from dislodged atheroma in the aortic arch; this is evidenced by the material captured within CEPs, which includes a mixture of calcium, tissue, thrombus, and atheroma. Subacute (24 hours to 30 days) stroke may be due to continued thrombus formation around smaller embolised material after the procedural anticoagulation has lost its effect or it may only be due to a delay in diagnosis by way of lingering procedural sedation masking stroke symptoms. Both subacute and early stroke (>30 days to 1 year) can be due to thrombus formation on the new valve (subclinical leaflet thrombosis [SLT]) in the setting of altered rheology, while stroke due to new-onset atrial fibrillation (NOAF) may also predominate in this time period. Late stroke (>1 year) after TAVI appears more related to frailty and atherosclerotic burden (e.g., chronic kidney disease [CKD], prior stroke) rather than procedural events, and the incidence of late stroke is likely not increased after TAVI compared to an adjusted general population rate4.
Clear predictors of stroke after TAVI have been difficult to ascertain. Current studies demonstrate inconsistencies among multivariable models, likely as a result of differences in the study populations (e.g., demographics, baseline risk) and low event rates that limit model precision. Further, inclusion of different groups of covariates will alter results (Table 3). A full list of proposed risk factors and their mechanism of stroke is presented in Table 4 and Table 5.
Table 3. Studies with multivariable analysis of predictors for TAVI-associated stroke.
| Author | Year | N | Study design | Stroke endpoint | Risk | Variables | Independent predictors of stroke |
|---|---|---|---|---|---|---|---|
| Nombela-Franco et al | 2012 | 1,061 | Retrospective | 30 days | High | Clinical, procedural | BPD, valve embolisation, NOAF |
| Samim et al | 2015 | 42 | Prospective | SBI | Int-high | Clinical, procedural, antithrombotics | Age, hyperlipidaemia, BPD, peak AVG |
| Auffret et al | 2016 | 72,318 | Meta-analysis | 30 days | Int-high | Clinical, CT, procedural | Female, NOAF, early experience, BPD |
| Kapadia et al | 2016 | 1,521 | RCT | 30 days | High | Clinical, procedural | Pre-TAVI AVG |
| Kleiman et al | 2016 | 3,687 | RCT | 10 days | High-extreme | Clinical, imaging, procedural | Apical access, prior CVA/TIA, angina, low BMI, recent fall, procedure time, rapid pacing, repositioning |
| Spaziano et al | 2018 | 537 | Prospective | 30 days | Int-high | Clinical, CT, procedural | LVOT calcium, baseline AF |
| Vlastra et al | 2019 | 10,982 | Retrospective | 30 days | Int-high | Clinical, procedural | Prior stroke, GFR <30 mL/min, NOAF |
| De Backeret al | 2020 | 2,455 | Retrospective | 90 days | Int-high | Clinical, antithrombotics | OAC naïve with or without AF (known AF on OAC protective therapy) |
| Matsuda et al | 2020 | 14,589 | Meta-analysis | 30 days | Low, int, high | Clinical, procedural | STS score, low-risk pop: SAVR+ (vs TAVI) |
| Pollari et al | 2020 | 581 | Retrospective | 30 days | Int-high | CT | LVOT calcium and RCC calcium, AF, urgency |
| De Carlo et al | 2020 | 117 | Prospective | SBI | Int-high | Clinical, procedural, CT, antithrombotics | Age-related white matter score, SEV and mechanically expandable valves, diabetes causes more gliotic scar after SBI |
| Woldendorpet al | 2021 | 2,171 | Meta-analysis | SBI | Int-high | Clinical, procedural | Diabetes, chronic renal disease, 3-Tesla MRI, and predilation |
| Maier et al | 2022 | 1,365 | Retrospective | 30 days | Int-high | Clinical, procedural | Prior stroke, large AVA (>0.55 cm²), large aortic angle (≥48.5°), RCC calcium, LVOT calcium, aortic arch calcium |
| Foley et al | 2022 | 433 | Retrospective | 30 days | Int-high | Clinical, CT, procedural | Aortic valve calcium, PAD, procedure time |
| Eschenbachet al | 2022 | 1,919 | Retrospective | 30 days | Int-high | Clinical, procedural | Prior stroke, operator experience |
| Linder et al | 2022 | 3,164 | Retrospective | 30 days | Int-high | Clinical, procedural, antithrombotics, CEP | Spontaneous echo contrast, reduced EF, CHA2DS2-VASc |
| Apor et al | 2022 | 153 | Prospective | WMH | Unknown | Clinical, procedural, antithrombotics | Hypoattenuated leaflet thrombosis |
| AF: atrial fibrillation; AVA: aortic valve area; AVG: aortic valve gradient; BMI: body mass index; BPD: balloon post-dilation; CEP: cerebral embolic protection; CT: computed tomography; CVA: cerebrovascular accident; EF: ejection fraction; GFR: glomerular filtration rate; int: intermediate; LVOT: left ventricular outflow tract; MRI: magnetic resonance imaging; NOAF: new-onset atrial fibrillation; OAC: oral anticoagulant; PAD: peripheral artery disease; pop: population; RCC: right coronary cusp; RCT: randomised controlled trial; SAVR: surgical aortic valve replacement; SBI: silent brain infarction; SEV: self-expanding valve; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack; WMH: white matter hyperintensity | |||||||
Table 4. Procedural risk factors for stroke in TAVI: mechanisms and evidence.
| Risk factor | Proposed mechanism | Magnitude of association | Strengthof evidence | Summary of evidence | Prevention |
|---|---|---|---|---|---|
| Procedure time | Dislodged valve debris by wire manipulation to cross; tight valve orifice that increases interaction with device; valve repositioning; alternative access; access site bleeding requiring cessation of baseline anticoagulation | + | Strong | Procedure time appears associated with stroke but encompasses a number of measured and unmeasured mechanisms | CEP, careful vascular closure technique |
| Alternative access | Selected patients with high atherosclerotic burden; access site determines interaction with the aortic arch | ++ | Strong | Transaxillary access is more associated with stroke than other access routes including transcaval and transcarotid | CEP, transcaval or carotid over axillary |
| Predeployment BAV | Predeployment BAV may dislodge valve debris | + | Neutral | Predeployment BAV was not associated with stroke in large observational studies but was a risk factor in the PROTECTED TAVR trial | Avoid routine use; if planned, consider CEP |
| Post-deployment BAV | Post-deployment BAV may still induce stroke, although native valve debris is in theory pinned by the THV | + | Strong | Multiple observational studies demonstrate association with stroke | Avoid routine use; if planned, consider CEP |
| THV design | SEV interaction with the ascending aorta or repositioning. Rapid pacing during BEV deployment induces period of hypoperfusion that may predispose the patient to stroke | Minimal/no effect | Strong | THV design has not been shown consistently to impact stroke rate. Difference in individual studies may be related to patient selection | - |
| Operator expertise | Increased volume improves adeptness with device therefore limiting undue interaction of the device with native anatomy | Minimal/ no effect | Neutral | Learning curves do not demonstrate that increased case volume reduces stroke rate | - |
| Valve-in-valve | Bioprosthetic valves with bulky or unstable calcium, or smaller annular area that increase device interaction causing debris embolisation | Minimal/no effect | Weak | No additional risk of stroke has been shown compared to native valve intervention, although sample size is limited with selected patients | Consider CEP |
| Leaflet modification | Dislodged valve debris by electrosurgical or mechanical leaflet cutting | + | Weak | Elevated risk of stroke among selected patients in a single-arm trial. CEP use encouraged in trial protocol | CEP |
| BAV: balloon aortic valvuloplasty; BEV: balloon-expandable valve; CEP: cerebral embolic protection; SEV: self-expanding valve; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | |||||
Table 5. Clinical risk factors for stroke in TAVI: mechanisms and evidence.
| Risk factor | Proposed mechanism | Magnitude of association | Strengthof evidence | Summary of evidence | Prevention |
|---|---|---|---|---|---|
| LVOT calcium | May represent more unstable calcium | ++ | Strong | Multiple CT studies demonstrate association with stroke | CEP |
| Calcium score | Greater burden of calcium indicates more potential debris | Minimal/no effect | Neutral | Calcium score alone does not reliably discriminate stroke occurrence | |
| Calcium pattern | Location of calcium may determine likelihood to embolise | Minimal/no effect | Weak | Aortic cusp or commissure location not consistently associated with stroke | |
| Bicuspid valve anatomy | Device underexpansion may lead to valve thrombus. Calcium burden is often high. Tendency for THV repositioning or adjunct balloon valvuloplasty | + | Strong | Among high-risk cases, bicuspid patients had more stroke than tricuspid patients, but this trend was not seen in intermediate-low-risk patients | CEP |
| Subclinical leaflet thrombosis | Altered valve rheology or inflammation leading to thrombus and subsequent embolisation | + | Strong | Meta-analysis suggests association with stroke. Relationship may be modified by burden of thrombus | Optimise valve geometry. CT imaging, AC |
| New-onset atrial fibrillation | NOAF induced by altered autonomics during the procedure or by inflammation induced by THV, if untreated predisposes to stroke | ++ | Strong | Meta-analysis suggests association with stroke. Pre-existing AF may be paradoxically protective | Pre-/post-procedure monitoring for AF with prompt AC |
| Surgical risk score | Marker of atherosclerotic burden and/or frailty | ++ | Strong | RCT data since the inception of TAVI shows overall correlation of periprocedural stroke with surgical risk score | CEP |
| Aortic arch atherosclerotic burden | Higher likelihood of dislodging debris during device advancement | ++ | Strong | Risk factors traditionally associated with atherosclerosis are associated with stroke, including age, surgical risk score, prior CVA, CKD, and age-related white matter score | CEP |
| Sex/gender | Women generally have higher risk for stroke than men, especially at an older age | + | Neutral | Despite lower comorbidity burden, women may have a marginally increased risk of stroke | CEP |
| Annular size | Small annuli increase risk of debris embolisation by heightened interaction between the device and native anatomy | + | Neutral | Older series show association of annular size with stroke | CEP |
| Carotid disease | Marker of aortic arch atherosclerotic burden or exacerbation of cerebral hypoperfusion by impedance of flow | Minimal/no effect | Neutral | Large data do not show association by severity of disease | - |
| Left atrial appendage thrombus | Source of embolism which may be provoked by wire manipulation, rapid pacing or arrhythmia during TAVI | + | Strong | Studies suggest elevated rate of stroke in patients with evidence of thrombus | CEP |
| AC: anticoagulation; AF: atrial fibrillation; CEP: cerebral embolic protection; CKD: chronic kidney disease; CT: computed tomography; CVA: cerebrovascular accident; NOAF: new-onset atrial fibrillation; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | |||||
Procedural risk factors
Procedural risk factors for stroke after TAVI are shown in Figure 2; their mechanisms, strength of evidence, and preventative measures are shown in Table 4.
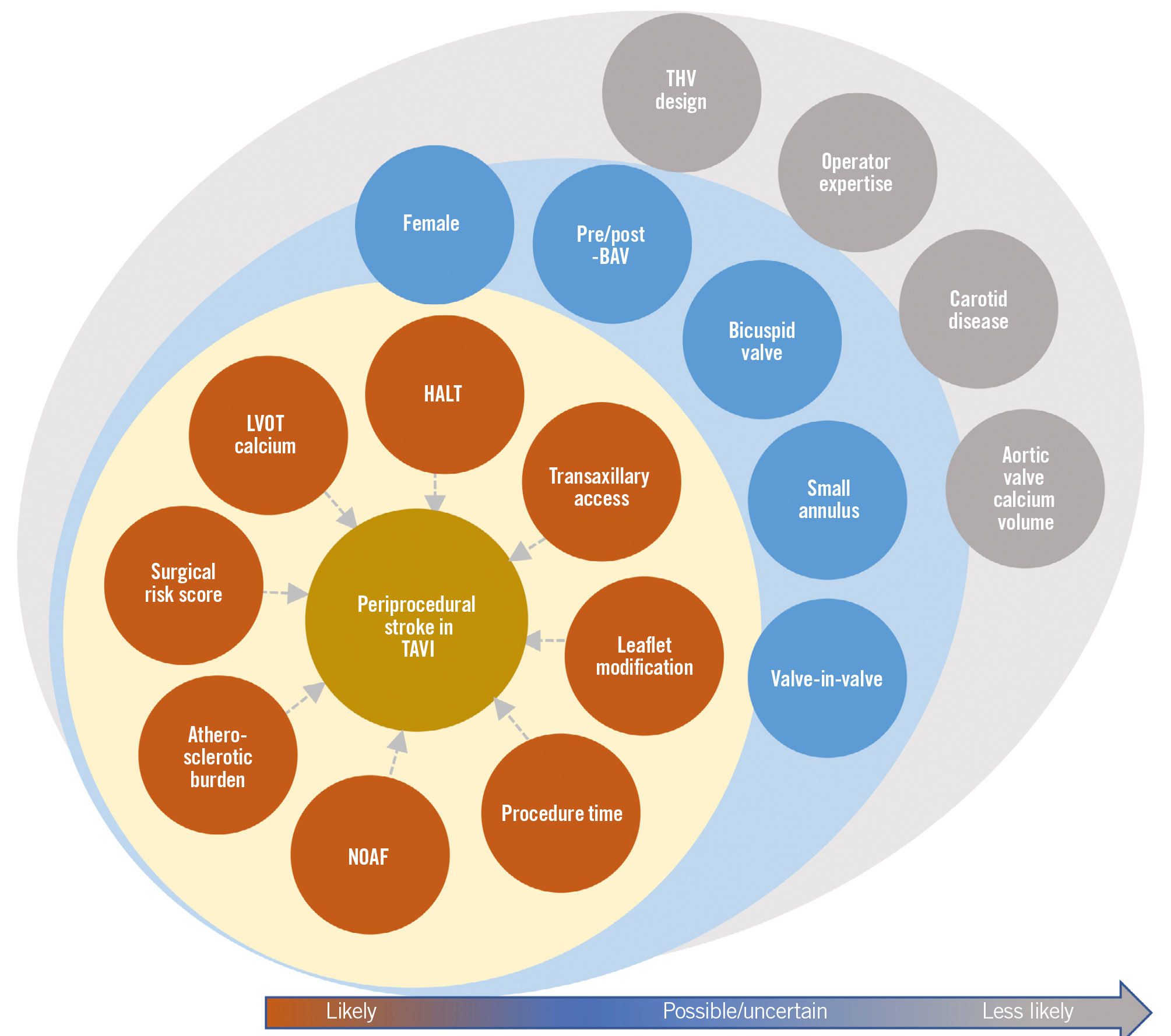
Figure 2. Risk factors for stroke with TAVI according to strength of evidence for association. BAV: balloon aortic valvuloplasty; HALT: hypoattenuated leaflet thickening; LVOT: left ventricular outflow tract; NOAF: new-onset atrial fibrillation; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
BALLOON AORTIC VALVULOPLASTY
Balloon aortic valvuloplasty (BAV), before or after valve deployment, may be utilised to optimise THV implantation. There is a theoretical risk that pre-TAVI BAV may dislodge debris from the native valve. However, at least two large observational trials found no difference between stroke rates in patients who had undergone pre-TAVI BAV versus direct TAVI10. The risk of debris embolisation with post-deployment BAV is thought to be less compared to pre-TAVI BAV, as potential embolic debris from the native valve is pinned by the implanted transcatheter valve. Observational studies have not demonstrated a consistent signal for harm due to post-TAVI BAV1112. Of note, potential confounders of this effect include anatomical attributes that both raise stroke risk and hinder THV expansion, prompting the need for post-TAVI BAV, e.g., leaflet or left ventricular outflow tract (LVOT) calcium, bicuspid valves, or ViV procedures.
TRANSCATHETER HEART VALVE TYPE
Differences in THV design and deployment process may lead to differential incidence and mechanisms of stroke by valve type.
The entirety of current clinical evidence suggests there is largely no difference in stroke rate between THV designs. Two recent meta-analyses of head-to-head valve comparisons found a slightly increased risk of stroke with balloon-expandable valves (BEVs) over self-expanding valves (SEVs), while the PROTECTED TAVR trial, which employed stratified randomisation by valve type, found that the use of BEVs was inversely associated with stroke incidence1314. The differences observed in stroke rates between THV platforms may be the result of confounding by factors that biased patient selection toward the valve used (e.g., LVOT calcium, annulus size, operator experience).
ALTERNATIVE ACCESS
Stroke rates for the different alternative access routes are shown in Table 6. Trans-subclavian/axillary access is the most common alternate access route, but this has been shown to have a higher risk of stroke compared to transfemoral access115. This may be related to the unfavourable angle of entry from the axillary artery into the aorta, which increases the risk of dislodged aortic plaque or aortic dissection, both of which can lead to stroke. Transcaval access, which avoids instrumentation of the head and neck vessels, has demonstrated lower stroke rates than transaxillary access16. Transcarotid access may also limit stroke compared to axillary access by granting more direct, in-line access to the aortic valve and because the surgeon is able to clamp the ipsilateral carotid artery during THV deployment followed by washout/de-airing before removing the clamp, similar to carotid endarterectomy.
Table 6. Stroke rate of alternative access routes for TAVI.
| Author | Transfemoralstroke | Transaxillarystroke | Transcavalstroke | Transcarotidstroke | Transthoracicstroke |
|---|---|---|---|---|---|
| Palmerini et al (2023) | 2.8 (518) | 5.9 (547) | - | - | 2.3 (642) |
| Lederman et al (2022) | 1.7 (7,132) | 13.2 (106) | 2.5 (238) | - | - |
| Carrol et al (2021)† | 2.3 (72,991) | - | - | - | - |
| Allen et al (2022) | - | - | - | 4.3 (667) | 3.7 (1,334) |
| Ooms et al (2021) | - | 5.7 (35) | - | - | - |
| Bob-Manuel (2020) | - | - | - | 5 (1,035) | - |
| Kirker et al (2020) | - | 7.4 (3,102) | - | 4.2 (801) | - |
| Debry et al (2020) | - | 3.2 (128) | - | 6.8 (374) | - |
| Costa et al (2020) | - | - | 2 (50) | - | - |
| Dahle et al (2019) | - | 6.3 (1,249) | - | - | 3.1 (2,379) |
| Greenbaum et al (2017) | - | - | 5 (100) | - | - |
| Weighted mean stroke rate | 2.3 | 6.9 | 3.1 | 4.9 | 3.2 |
| Values are % (n). The summary of studies is not comprehensive but includes recent studies reporting stroke rate for alternative access routes. †Stroke rate is shown for TAVI performed in 2019 from the Transcatheter Valve Therapy Registry. TAVI: transcatheter aortic valve implantation | |||||
Clinical risk factors
Clinical risk factors for stroke after TAVI are shown in Figure 2; their mechanisms, strength of evidence, and preventative measures are shown in Table 5.
ANATOMICAL FEATURES
CALCIUM BURDEN
A heavy calcium burden on native valves may plausibly lead to a higher risk of embolic stroke during TAVI; however, current evidence does not clearly support this notion. While aortic valve calcium volume has been correlated with the burden of microembolisation seen on transcranial Doppler ultrasound during TAVI17, it is unlikely that calcium volume alone predicts periprocedural stroke after TAVI in a meaningful way18. Furthermore, the effect of CEP on stroke rate was not modified by aortic valve calcium volume in the PROTECTED TAVR trial12. Other studies, however, have shown that LVOT calcium, which may represent more unstable calcium, does increase risk of stroke19. Other calcium patterns (cusp or commissural distribution) have not consistently been shown to be associated with increased stroke risk19.
BICUSPID VALVE
The unique aspects of bicuspid anatomy may contribute to an increased risk of stroke during TAVI procedures. Factors such as heavy leaflet calcification, the presence of a raphe, or stiff leaflets can result in poor THV expansion, potentially triggering hypoattenuated leaflet thickening (HALT). Additionally, a high calcium burden may elevate the risk of embolisation, and uncertainty regarding the annular plane due to asymmetric cusps may also entail multiple THV recaptures or repositioning. An analysis from the STS database revealed a significantly higher 30-day stroke rate among bicuspid TAVI patients compared to those with tricuspid anatomy (2.5% vs 1.6%; hazard ratio [HR] 1.57, 95% confidence interval [CI]: 1.06-2.33)7. However, in a subsequent analysis focusing solely on low surgical risk patients, the 30-day stroke rate was similar between the two groups (1.4% vs 1.2%, HR 1.14, 95% CI: 0.73-1.78)20.
SUBCLINICAL LEAFLET THROMBOSIS
SLT or HALT can occur after TAVI and may increase the risk of subsequent stroke; however, there is conflicting evidence. A recent comprehensive meta-analysis found that SLT, occurring in 6.0% of patients after TAVI and predominantly in those receiving SAPIEN BEVs (Edwards Lifesciences), conferred a 2.6-fold increased risk of stroke at follow-up21. One small study (n=91) also found that SLT was associated with an increased volume and frequency of white matter hyperintensities − a component of SBI − on MRI performed 6 months post-TAVI22. Conversely, two recent meta-analyses did not show an association of SLT with neurological events2324.
NEW-ONSET ATRIAL FIBRILLATION
NOAF may occur after TAVI and has been associated with adverse outcomes including stroke and death. The causative mechanism of NOAF after TAVI is likely multifactorial, occurring in the setting of predisposing comorbidities (e.g., age, CKD) and procedural events that provoke inflammation, enhanced sympathetic tone, and oxidative stress. Due to the nature of NOAF after TAVI and the time needed for cardiac thrombus formation, stroke-related NOAF tends to predominate in the subacute period (between 1 and 30 days)25. It is important to note that NOAF may confer a higher risk of stroke compared to pre-existing atrial fibrillation (AF), indicating a possible protective effect of pre-existing AF related to oral anticoagulant (OAC) use26. Further studies are needed to investigate whether OACs may modify the relationship of NOAF with stroke incidence.
Prevention
Preventing stroke in TAVI patients has been difficult to achieve, and it remains unclear which strategies are effective to reduce periprocedural stroke. The debris captured in CEP devices offers insights into the origins of stroke in TAVI and may inform on potential targets for prevention. Filter baskets may contain thrombus, calcification, valve tissue, artery wall fragments, and foreign material. This underscores the importance of careful device manipulation within the native valve and traversal of the aortic arch. The presence of thrombus may indicate a cause of stroke that is readily modifiable. Despite adequate anticoagulation, sources of thrombus may still include large-bore sheaths, catheters, and reaction to the THV prosthesis leading to thrombosis on the leaflets. In addition, while CEP devices might be able to prevent intraprocedural embolisation, thrombus formation may occur after the procedure due to HALT or NOAF or around the nidus of previously embolised material in the brain once intraprocedural anticoagulation has washed out. Recommendations for postprocedural monitoring and antithrombotic regimens are discussed herein.
Postprocedural monitoring
The majority of TAVI-associated stroke occurs within 30 days of the procedure. While stroke in the acute periprocedural period is often related to procedural events, the mechanism for stroke during the subacute period may be due to SLT or NOAF. Postprocedural ambulatory electrocardiogram (AECG) monitoring for AF has been shown to detect more AF events, although translation to a reduction in stroke rates is yet to be realised27. Cost-effectiveness may limit the broad application of AECG, but it could be selectively applied in patients at the highest risk for NOAF, i.e., those with advanced age or a high risk score. Smartwatch detection may be a more practical alternative28.
SLT is infrequently screened for outside of controlled trials, but it is not rare in the periprocedural period (~6% at 30 days)21. Although anticoagulation can effectively resolve SLT, SLT occurrence is difficult to predict, and screening with computed tomography (CT) imaging for all patients is not practical, while transthoracic echocardiography has limited sensitivity. Furthermore, SLT may be dynamic in that it can come and go at different timepoints within a year after TAVI, as shown in a CT substudy of the PARTNER 3 trial29; this likely confounds management paradigms to reduce SLT-related stroke. An elevated prosthetic valve gradient at 30-day echocardiography or symptoms suggestive of transient ischaemic attack may prompt CT imaging, or prophylactic anticoagulation may be used in select cases, such as low-risk patients30. Of note, current European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) and American College of Cardiology (ACC)/American Heart Association (AHA) guidelines give a class IIb recommendation for anticoagulation with a vitamin K antagonist 3-6 months after TAVI in low-risk patients3132.
Antithrombotic therapy
INTRAPROCEDURAL ANTITHROMBOTICS
Currently, unfractionated heparin is the mainstay of thromboembolic management, with an activated clotting time goal of >250 seconds. Bivalirudin has not been shown to be superior to heparin in this setting and is costlier33. The use of protamine appears safe to reduce vascular access complications and bleeding without increasing ischaemic events34.
POSTPROCEDURAL ANTITHROMBOTICS
In the early stages, dual antiplatelet therapy (DAPT) was recommended after TAVI for all patients without an indication for anticoagulation. However, subsequent studies found increased adverse events with DAPT compared to single antiplatelet therapy (SAPT). SAPT after TAVI is currently supported by ESC/EACTS 2021 (class I) and ACC/AHA 2020 (class IIa) guidelines3132.
Consideration of anticoagulation after TAVI was derived from the surgical literature in which an initial course of anticoagulation is meant to mitigate any innate reaction to prosthetic material until endothelisation can occur. Although anticoagulation after TAVI might be expected to reduce stroke related to valve thrombosis or NOAF, its routine use has not been shown to reduce embolic events for patients without an indication for anticoagulation and may cause harm35.
Cerebral embolic protection devices: current state and future directions
Use of CEP devices during TAVI has increased rapidly in recent years36; however, CEP device use is still not universal, largely because the available evidence for its efficacy is equivocal. The PROTECTED TAVR trial did not unequivocally demonstrate stroke reduction with the use of SENTINEL (Boston Scientific); however, there remains potential benefit from the use of CEP devices. One cause for continued interest is that the rate of disabling stroke appeared reduced in the SENTINEL arm, albeit the trial was not powered for this endpoint. This trend may be attributed to the occurrence of incomplete device sealing in certain patients, allowing the passage of small particles (resulting in persistent non-disabling strokes), while larger particles are still effectively blocked (leading to fewer disabling strokes). However, while this mechanism may plausibly reduce particle size, it does not account for the significant role of stroke location in determining the severity of disability37. In line with this, the CLEAN-TAVI Trial, which evaluated MRI brain findings after TAVI with and without the use of SENTINEL, revealed comparable lesion distribution between groups, albeit with lower lesion volume in the SENTINEL group38. It is also critical to note that the primary outcome of PROTECTED TAVR was negative, and therefore, caution is advised when interpreting a secondary, non-powered endpoint. Specifically, the observed reduction of disabling stroke may be attributable to a type 1 error or multiplicity, potentially resulting in a chance finding. Additionally, the 95% CI for the primary outcome (−1.7 to 0.5) also indicates the possibility of harm from using SENTINEL, and it is plausible that manipulation of the device in the brachiocephalic or carotid arteries or the aortic arch could introduce an iatrogenic source of embolisation. Nevertheless, the BHF PROTECT-TAVI trial, which aims to enrol 7,730 patients, has a similar trial design to PROTECTED TAVR, and the planned meta-analysis of the two trials (PROSPERO Registry number: CRD42022324160) should elucidate the effect of SENTINEL on the prevention of stroke during TAVI.
Current CEP devices also leave gaps regarding the mechanism of protection. SENTINEL, the only device approved in the USA, is introduced via the right radial artery but does not cover the left vertebral artery. Any reduction in stroke with SENTINEL may be offset by iatrogenic embolisation from device placement in the arch vessels. TriGUARD 3 (Keystone Heart; not currently approved in the USA), a device which provides full arch coverage, introduced via the contralateral femoral artery, was studied in the REFLECT II trial, and it was found that the device had incomplete arch vessel coverage in 40% of cases. In addition, bleeding and vascular complications were increased with use of the device, which was attributable to the need for larger-calibre femoral access39. Several devices currently in trials or in development may address some of the shortcomings of these CEP devices (Table 7). Those that avoid carotid instrumentation and provide reliable full arch coverage, with lower operator dependence, may prove most effective, although it is important for there not to be a trade-off with increased vascular access complications and bleeding due to the need for larger-calibre contralateral femoral artery access.
Table 7. Embolic protection devices.
| Device | Regulatory status | Ongoing trials | Coverage | Access site | Sheath size | Pore size | Mechanism |
|---|---|---|---|---|---|---|---|
| Emblok | Investigational | European Study (NCT03130491) | Full arch | Femoral | 11 Fr | 125 μm | Capture |
| Emboliner | Investigational | ProtectH2H (NCT05684146) | Full body | Femoral | 9 Fr | 150 μm | Capture |
| FLOWer | Investigational | NAUTILUS (NCT04704258) | Full body | Femoral | 12 Fr | 65 μm | Capture |
| POINT-GUARD | Investigational | CENTER | Full arch | Femoral | 10 Fr | 105 μm | Deflection |
| ProtEmbo | Investigational | PROTEMBO SF (NCT03325283) | Full arch | Radial | 6 Fr | 60 μm | Deflection |
| SENTINEL | CE mark/U.S. FDA approved | BHF PROTECT-TAVI (ISRCTN16665769) | BCT, LCCA | Radial | 6 Fr | 140 μm | Capture |
| TriGUARD 3 | CE mark/ investigational | - | Full arch | Femoral | 8 Fr | 115 μm | Deflection |
| Emblok (Innovative Cardiovascular Solutions); Emboliner (Emboline); FLOWer (AorticLab); POINT-GUARD (Transverse Medical); ProtEmbo (Protembis); SENTINEL (Boston Scientific); TriGUARD 3 (Keystone Heart). BCT: brachiocephalic trunk; CE: European Conformity; FDA: Food and Drug Administration; Fr: French; LCCA: left common carotid artery | |||||||
Future considerations for trial design in stroke prevention
The slightly reduced rate of stroke after TAVI in contemporary data is likely attributable to a lower overall risk profile of the TAVI population and, in part, to the miniaturisation of THV designs that were developed for safer vascular access or successful valve deployment. However, adjunct measures to reduce stroke after TAVI have yet to show a definitive benefit. Given the mortality and morbidity attached to stroke (Figure 3), a more focused effort to reduce events is needed from industry and investigators alike. The first step to reducing stroke after TAVI is to properly identify the TAVI population at the highest risk for stroke; this will serve to allow the enrichment of study populations in CEP device trials, improving the power to detect a true benefit while also reducing costs. Further, improved understanding of TAVI-associated stroke mechanisms and the correlation between stroke localisation and severity will facilitate THV and CEP device innovation towards achieving a reduction in events. Prediction models for stroke after TAVI have proved challenging to develop, primarily because of variations in study populations and the covariates considered in modelling. Traditional logistic modelling is also limited in this regard given the rarity of stroke events and the complex multifactorial nature that may not be captured by logistic models alone. The emergence of artificial intelligence technologies offers promise in this area. For example, a recent study utilised deep learning and neural networks to incorporate over 100 clinical, anatomical, and procedural risk factors, resulting in a model with moderate predictive ability for stroke after TAVI. Nevertheless, prospective validation in larger cohorts is warranted to confirm these findings40.
The outcomes of the ongoing SENTINEL trials will have a profound influence on future clinical investigations involving CEP. If these trials yield positive results, it is highly probable that the device’s indication for use would transition beyond its current purpose of “capturing and removing thrombus/debris” to also include the prevention of stroke during or immediately after TAVI. This, in turn, would further galvanise the CEP field, and several CEP devices may be compared to SENTINEL. Accurate determinations of efficacy for investigational CEP devices will depend on the standardisation of event detection, which notoriously varies with methods of ascertainment2. Following the Neurologic Academic Research Consortium (NeuroARC) guidelines for trial design would maximise event detection, therefore improving the sample power while also permitting reliable comparisons between trials. Thus, dedicated neurological assessment before and after intervention should be mandatory, with DWI being the norm in CEP trials. Finally, the interaction of CEP with SBI and neurocognitive decline is uncertain and warrants additional investigation, especially as TAVI is increasingly performed in younger and lower-risk patients.
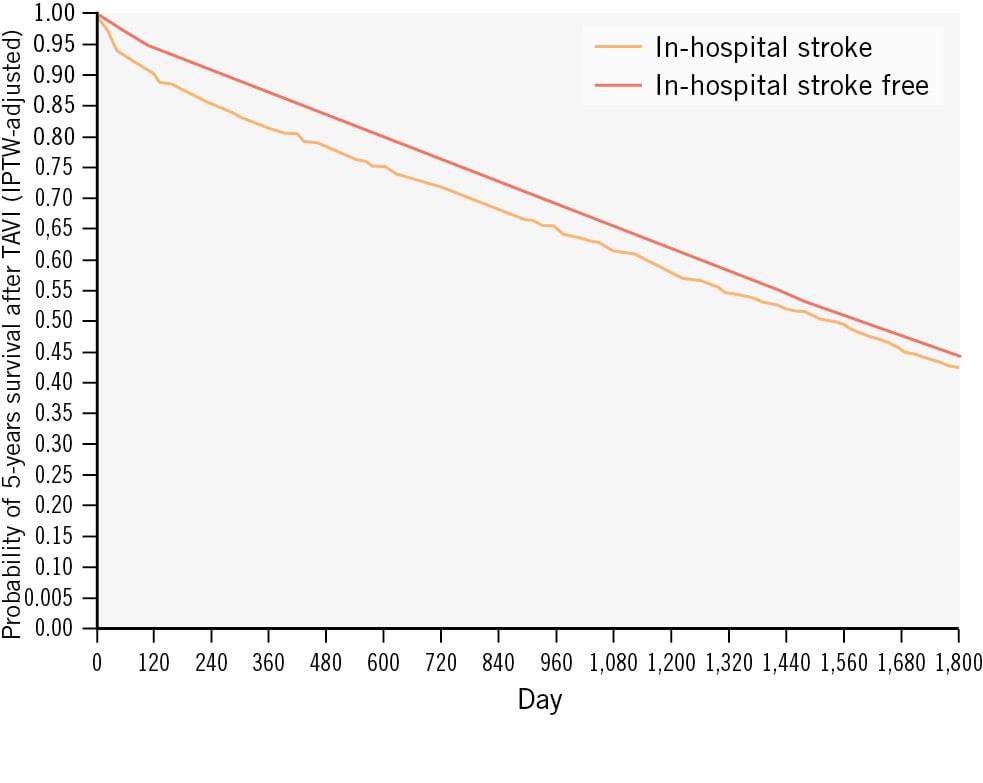
Figure 3. Stroke after TAVI and its association with mortality41. IPTW: inverse probability of treatment weighting; TAVI: transcatheter aortic valve implantation
Conclusions
Despite advancing technology, cerebrovascular events after TAVI remain a significant concern. Considerable investigation is still needed to understand which patients are at the highest risk, not only to help guide the use of CEP devices but also to properly inform patients of the stroke risk associated with their procedure, particularly as younger patients with potentially less severe aortic stenosis are considered for intervention. Larger trials evaluating the efficacy of CEP are on the horizon, and a panoply of new devices under clinical investigation suggest that the future of CEP is bright and it may indeed become routine in the near future. If this does transpire, then post-marketing surveillance will be paramount to ensure that we are, in fact, utilising devices that are safe, without increased stroke events, or bleeding complications that offset the ischaemic benefits.
Conflict of interest statement
T. Rogers - consultant: Edwards Lifesciences, Medtronic, and Boston Scientific; advisory board: Medtronic and Boston Scientific; equity: Transmural Systems; intellectual property: co-inventor on patents, assigned to NIH. R. Waksman - advisory board: Abbott, Boston Scientific, Medtronic, Philips IGT, and Pi-Cardia Ltd.; consultant: Abbott, Append Medical, Biotronik, Boston Scientific, JC Medical, MedAlliance/Cordis, Medtronic, Philips IGT, Pi-Cardia Ltd., Swiss Interventional/SIS Medical AG, and Transmural Systems; institutional grant support: Biotronik, Medtronic, and Philips IGT; investor: Transmural Systems. The other authors have no conflicts of interest to declare.

