Abstract
Transcatheter aortic valve implantation (TAVI) is an evidence-based treatment alternative for selected high-risk patients with symptomatic severe aortic stenosis as acknowledged in the most recent edition of the ESC Guidelines on Valvular Heart Disease 2012. However, periprocedural complications and in particular cerebrovascular accidents remain a matter of concern. While transcatheter heart valve technology continuously improves and the development of novel and even less invasive implantation techniques is on-going, cerebrovascular events complicating TAVI may abrogate the usual improvement in terms of prognosis and quality of life. This article describes the incidence of cerebrovascular events after cardiovascular procedures, provides an overview of the pathophysiological mechanisms as well as the impact on outcomes and provides some insights into preventive strategies as well as the acute management of these events.
Introduction
Cerebrovascular accidents (CVA) complicating cardiovascular therapeutic interventions are among the most feared adverse events from a patient’s perspective. Depending on the extent and location of affected brain tissue, CVAs are associated with impaired survival and considerable morbidity due to partial or complete loss of independence in everyday activities. This article will focus on CVAs among patients undergoing transcatheter aortic valve implantation (TAVI) and will provide an overview of the incidence, diagnosis, pathophysiology, and preventive strategies as well as acute management of CVAs in this context.
Frequency, timing and risk factors
SPONTANEOUS RISK OF STROKE
CVAs are the fourth leading cause for mortality after heart disease, cancer and death from chronic lower respiratory disease, and account for one of every 18 deaths in the United States1. While the annual incidence of stroke has diminished in developed countries, presumably as a result of improved risk factor modification2, the total number of strokes continues to increase worldwide3. The prevalence of CVA is age-dependent with a rate as low as 0.3% among men and 0.5% among women in the age group of individuals between 20 and 39 years of age and as high as 14.5% among men and 14.8% among women in the age group of individuals 80 years of age or older (Figure 1)1. This increase is attributed to the individual stroke risk profile, which comprises the presence of one or several risk factors, including arterial hypertension, female gender, diabetes mellitus, left ventricular hypertrophy, atrial fibrillation and smoking4. In view of the increasing life expectancy and the attendant risk of stroke, CVAs are recognised as being a significant healthcare problem5.
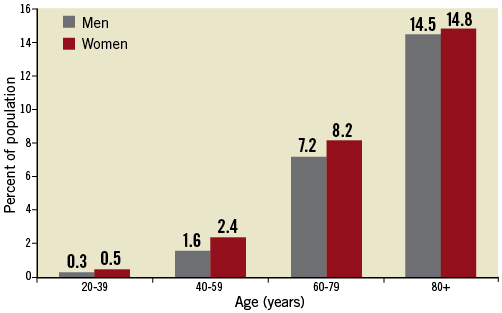
Figure 1. Prevalence of cerebrovascular accidents stratified for age and gender (National Health and Nutrition Examination Survey: 2005-2008).1
CARDIOVASCULAR THERAPEUTIC INTERVENTION - RELATED RISK OF STROKE
Several cardiovascular therapeutic interventions are associated with a certain risk of stroke. Numerous studies have pointed to the risk of perioperative stroke among patients undergoing cardiovascular surgery with an incidence of 1.4% to 3.8% for patients undergoing isolated coronary artery bypass grafting (CABG)6, 1% to 4% for patients undergoing isolated valve surgery7,8, 4% to 5% for patients undergoing combined CABG and valve surgery9-11, and the highest rate reported for patients undergoing double-valve or triple-valve surgery (9.7%)12. Most of these events are embolic in nature13, and have been largely attributed to intra-operative manipulation of the ascending aorta14. Surgical techniques have evolved over time in order to mitigate this risk and include off-pump or beating-heart surgery as well as alternative cannulation sites in order to avoid manipulation in the aortic arch, thereby reducing the risk of mobilisation of atherosclerotic debris15.
Percutaneous cardiovascular interventions are considered less invasive compared to surgical techniques. The risk of stroke among patients undergoing percutaneous coronary intervention is consistently lower compared with that of patients undergoing CABG and has a frequency of 0.4%16,17. Conversely, the risk of CVA among elderly patients undergoing isolated balloon aortic valvuloplasty has been between 1% and 2%18-21, and transcatheter aortic valve implantation (TAVI) has been associated with rates of periprocedural stroke in the range of 3% to 6% in observational studies (Figure 2)22-26. The risk of stroke among patients undergoing TAVI has been related to thromboembolic complications as a result of the calcified valvular apparatus17, passage of large bore catheters through the aortic arch and ascending aorta with the potential to dislodge atheroma, activation of platelets and the coagulation cascade as well as air embolism in cases of transapical access. The majority of CVAs occur during the early periprocedural period, with an early peaking hazard phase during the first week after the intervention27-30 followed by a constant late hazard27.
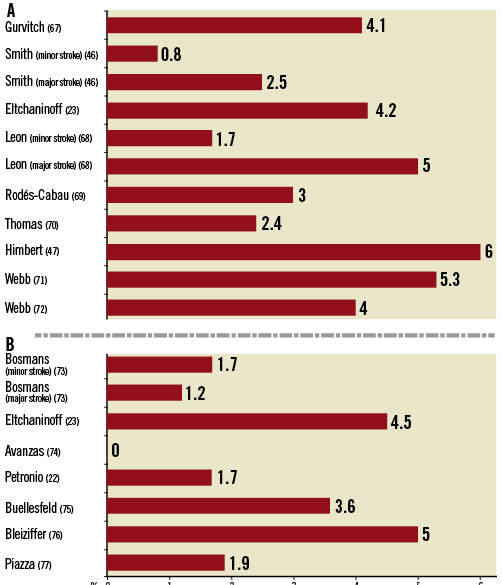
Figure 2. Cerebrovascular events after TAVI using the transfemoral access route according to device type. Panel A summarises the incidence of stroke in major studies using the Edwards SAPIEN bioprosthesis, while Panel B summarises the rates of stroke in major studies using the Medtronic CoreValve bioprosthesis within 30 days after the intervention.
In the randomised PARTNER A trial comparing TAVI with surgical aortic valve replacement (SAVR), TAVI was associated with a higher risk of the combined endpoint of stroke and TIA with rates of 4.6% (TAVI) vs. 2.4% (SAVR, p=0.12) at 30 days, 8.7% vs. 4.3% (p=0.03) at one year, and 11.2% vs. 6.5% (p=0.05) at two years27. More than 50% of events occurred during the first 10 days after the procedure, 58% of TAVI-related neurologic events were classified as major stroke compared with 69% of SAVR-related neurologic events, and more than 70% of CVAs were ischaemic in origin. During the periprocedural period, TAVI (rather than SAVR), and small aortic valve area, in the case of TAVI, were associated with an increased risk of stroke, whereas the long-term risk was determined by patient-related factors including previous history of stroke and advanced NYHA Class in both treatment arms.
Since the early TAVI experience, the risk of periprocedural complications including the risk of stroke has steadily decreased over time. Several reasons are responsible for this evolution:
First, the procedure has been simplified, standardised and is performed by operators who have overcome the initial learning curve28,29.
Second, screening of suitable patients for TAVI has largely improved and the decision as to the most appropriate treatment is typically based on consensus agreement among members of a multidisciplinary “Heart Team” taking into account perioperative risk as well as anatomical characteristics with special consideration given to periprocedural complications including stroke30.
Finally, delivery catheters and valve design underwent several iterations resulting in smaller delivery catheters, improved steerability of delivery catheters minimising interference with the aortic arch and ascending aorta, and lower device profile to cross the calcified annulus31.
DEFINITION OF STROKE IN THE CONTEXT OF TAVI
In the absence of standardised endpoint definitions, previous observational studies used different definitions for the assessment of adverse events including stroke, which at least in part explains differences in clinical outcome across various studies. In January 2011 the Valve Academic Research Consortium (VARC) provided a consensus document with endpoint definitions to standardise TAVI outcomes32. The uniform endpoint definition for CVA according to VARC takes into account the following considerations:
– Clinical symptoms, suggestive for a neurological event not considered to be from a metabolic or pharmacological origin ideally assessed by a neurologist
– Confirmation of the clinical diagnosis by neuroimaging, ideally diffusion-weighted MRI
– Assessment of functional severity of neurologic deficits
– Classification into categories of major stroke, minor stroke or transient ischaemic attack (TIA)
– Classification into haemorrhagic or ischaemic origin
The differentiation of CVAs into major stroke, minor stroke, or TIA has been largely based on the severity of the neurologic adverse event and related to the timing and results of neuroimaging assessment. While the diagnosis of a stroke requires sustained neurological symptoms and a positive neuroimaging study, a transient ischaemic attack (TIA) is characterised by an unremarkable neuroimaging study and transient symptoms with rapid and complete resolution within 24 hours. The severity of a stroke event is classified according to the expression of clinical disability using the modified Rankin Scale (mRS)33. Following the initial calculation of the mRS after seven days or at hospital discharge, the assessment of clinical disability is currently based on mRS performance at both 30 days and 90 days of follow-up. CVAs resulting in a mRS ≥2 are considered major strokes, whereas the diagnosis of minor strokes applies to patients with a mRS <2 at 30 days and 90 days of follow-up. Apart from the assessment of clinical disability, VARC recommends classifying CVAs into ischaemic, haemorrhagic or undetermined origin based on the results of neuroimaging. An updated definition of stroke is expected to be published in the revised second edition of the VARC initiative34.
DIAGNOSTIC MODALITIES TO ASSESS STROKE
Symptoms suggestive of CVA are usually of sudden onset but present with a myriad of possible clinical characteristics depending on the location and extent of affected brain tissue. Once the patient has been evaluated by an experienced specialist in clinical neurology, neuroimaging is required to determine the size and location of the defect and timely implementation of a treatment strategy. Cerebral tumours and subdural haematomas need to be excluded, and the distinction between intracranial haemorrhage and ischaemic cerebral infarction allows for further treatment stratification.
Non-enhanced multislice computed tomography images (MSCT) primarily serve to exclude intracranial haemorrhage. The ubiquitous and fast availability, the lack of major exclusion criteria as well as the rapid scan time are the principal advantages of MSCT in the assessment of acute CVA and this is supported by current guidelines35. Compared with MSCT, neuroimaging with the use of magnetic resonance imaging (MRI) has additional advantages. The early detection of ischaemia and of small cerebral defects is particularly useful among patients presenting with acute stroke within the eligible time frame to undergo intravenous thrombolysis. Moreover, the accuracy to differentiate between cerebral haemorrhage and ischaemia is similar to MSCT36, which makes MRI the preferred imaging modality to evaluate patients with acute stroke35. The disadvantages of MRI remain the longer scan time and the more frequent exclusion criteria such as permanent pacemakers or internal cardioverter defibrillators.
PATHOPHYSIOLOGY OF TAVI-RELATED STROKE
In contrast to the rate of clinically apparent CVAs among patients with severe aortic stenosis undergoing TAVI, several studies observed evidence of clinically silent cerebrovascular injury in a considerable number of patients. Using diffusion-weighted MRI at baseline, shortly after the procedure and at three months follow-up, Kahlert and colleagues detected hyperintense lesions in 80% of patients treated with the Medtronic CoreValve (Medtronic, Inc., Minneapolis, MN, USA) and 86% of patients treated with the Edwards SAPIEN TAVI prosthesis (Edwards Lifesciences, Irvine, CA, USA)37. When comparing the results to a patient population undergoing SAVR, fewer patients were found to have neuroimaging-defined evidence of new lesions with the latter intervention (SAVR 48%, p=0.01), although the volume of individual defects was larger (77 [59 to 94] vs. 224 [111 to 338] mm3; p<0.001).
The hypothesis of a primarily embolic origin is further supported by previous evidence suggesting an association of left-sided cardiac annular and valvular calcification with an increased rate of cerebrovascular tissue injury38, and a correlation of aortic valve sclerosis with clinically apparent stroke as well as all-cause mortality39. Apart from the spontaneous risk of cerebral injury related to the underlying aortic valve disease, the TAVI procedure itself is associated with an increased risk of thromboembolism. Instrumentation in the ascending aorta, the retrograde passage of the native aortic valve17,40, balloon inflation within the stenosed aortic valve, delivery of the transcatheter heart valve assembly through the aortic arch, and the deployment of the prosthesis itself are individual steps which may contribute to the risk of thromboembolism. Evidence from transcranial Doppler ultrasonography (TCD) studies suggests that the majority of high-intensity transient signals (HITS) occur during the deployment of the prosthesis41. These findings need to be interpreted with caution, as HITS may represent solid particles responsible for new cerebral injury, but may also reflect gaseous material without clinical relevance.
The individual periprocedural risk of CVA is related to the age of the patient, the severity of aortic valve stenosis and the presence and extent of aortic arch atheroma42,43. The prevalence of both severe aortic valve stenosis and significant aortic arch atheroma is as high as 54%, which increases even further to 61% among patients above the age of 65 years44. Currently, there is no evidence to suggest differences in the risk for stroke between the two most frequently used TAVI prostheses, the balloon-expandable Edwards SAPIEN and the self-expanding Medtronic CoreValve (Figure 2).
TAVI performed via the transapical access route is the only approach allowing for an antegrade passage of the aortic valve, potentially reducing the risk related to guidewire manipulation in the ascending aorta and the aortic arch. However, new intracranial defects as detected by diffusion-weighted MRI have been observed as frequently as with TAVI procedures performed via other access routes requiring retrograde passage of the native aortic valve45, without differences in size and distribution of cerebral infarcts. Similarly, there were no differences in the risk of stroke between patients undergoing transapical TAVI compared with conventional SAVR in the PARTNER A cohort46. Accordingly, transapical TAVI cannot be considered a technique associated with a lower risk of stroke although differences in baseline risk between patients undergoing transfemoral compared with transapical TAVI need to be considered.
PROGNOSTIC IMPACT OF STROKE AFTER TAVI
In general, stroke is the most common cause of dependence in activities of daily living and is associated with an increased risk of morbidity and mortality. The degree of physical and mental disability after stroke correlates with the location and extent of infarcted brain tissue. In contrast to minor CVAs with complete recovery of neurologic function47 , disabling stroke resulting in serious physical disability has been associated with impaired survival at short-term (30 days) (Figure 3)50 and longer-term follow-up (one year) after TAVI compared with patients without neurological events (66.7% vs. 27.7%, p<0.0001)48. A recent systematic review of 10,037 patients undergoing TAVI reported a risk of stroke of 3.3% at 30 days and of 5.2% at one year after TAVI. Patients suffering from stroke faced a more than threefold increased risk of impaired clinical outcome compared to event-free patients49. Moreover, mortality after stroke remained at an increased level throughout one year among patients undergoing TAVI by the transfemoral access route, while patients undergoing TAVI by transapical access continued to have an increased risk of mortality beyond the first year in the PARTNER A trial27.
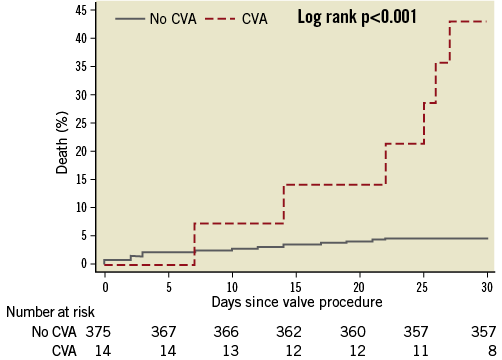
Figure 3. Cumulative incidence of all-cause mortality among patients with and without cerebrovascular accidents within 30 days after transcatheter aortic valve implantation. Patients without cerebrovascular event (blue line, no CVA) and patients with cerebrovascular events (red line, CVA). Obtained with permission50.
PREVENTION OF CEREBROVASCULAR INJURY
The majority of clinical and subclinical CVAs after TAVI occur during the early periprocedural period within 48 hours after the procedure, suggesting a thromboembolic origin. Moreover, there is a growing body of evidence suggesting that post-valve-deployment balloon dilatation and repeated prosthesis placement attempts in case of self-expanding heart valves are associated with an increased risk of stroke45,50,51. Several implantation techniques have been proposed to avoid cerebral embolic injury, but data on the effectiveness are scarce. A “no-touch” implantation technique was introduced in a case report52. Severe atherosclerotic disease with significant atheroma in the aortic arch and the ascending aorta was prohibitive to performing transfemoral TAVI and a transapical implantation strategy was chosen, thereby avoiding wire manipulation in the ascending aorta, with successful performance of TAVI. More recently, a pilot study introduced the concept of performing TAVI without prior balloon dilatation53. As any manipulation in the ascending aorta and contact with the severely calcified native aortic valve increases the potential for thromboembolism, this technique may minimise the risk associated with prior balloon aortic valvuloplasty. However, this technique may not be applicable to all patients undergoing TAVI, may require post-dilation of the prosthesis refuting the original premise, may be limited to patients undergoing TAVI with self-expanding prostheses, and requires validation in appropriately designed studies.
The concept of reducing cerebral embolism during cardiac surgery by the use of dedicated mechanical cerebral protection devices has been adapted and redesigned for TAVI (Table 1)54. Several cerebral protection devices with different mechanisms of action have been introduced and are currently under investigation. The Embrella protection device (Edwards Lifesciences, Irvine, CA, USA)55 as well as the SMT Shimon Embolic protection Filter™ (SMT Research and Development Ltd, Herzliya, Israel)56 are designed to shield the supraaortic vessels with a filter membrane placed in the aortic arch to deflect embolised debris. The Embrella deflection device is delivered through a long vascular sheath via the right radial or brachial artery and covers the origin of the left carotid and innominate artery after deployment of its polyurethane membrane (100 µm filter) in the ascending aorta.
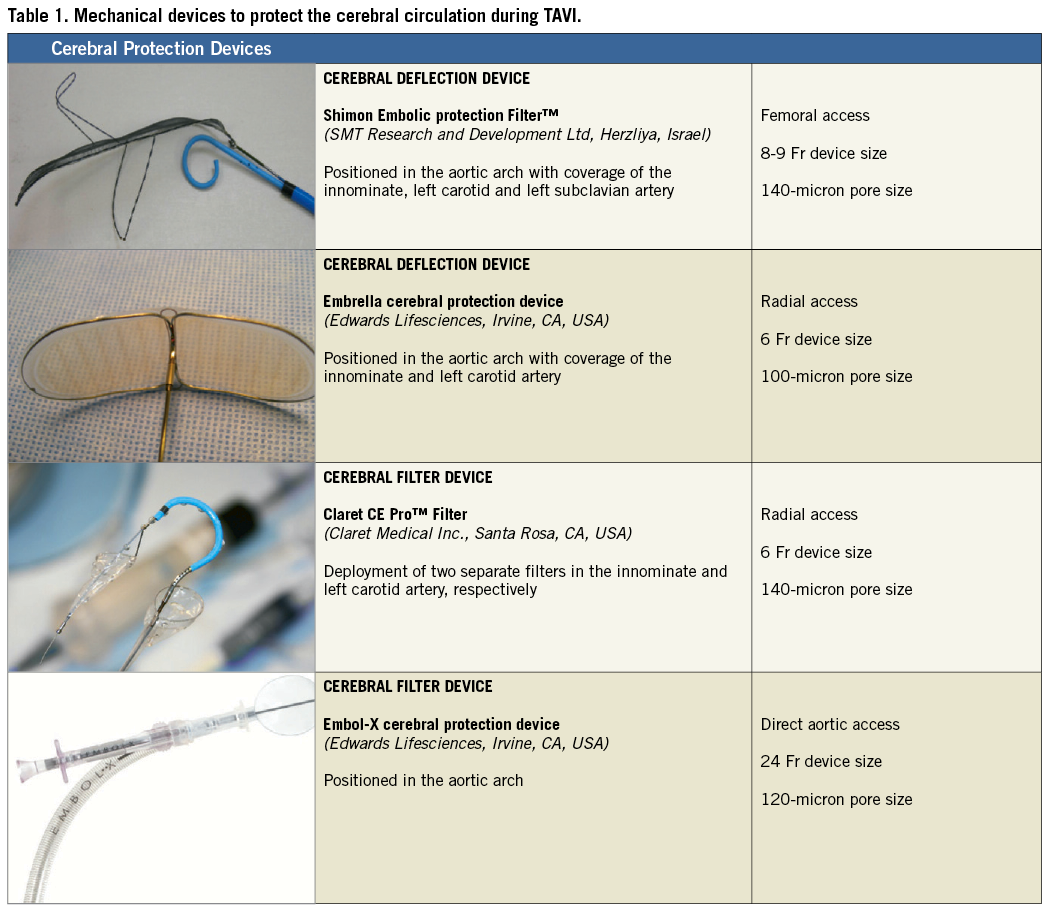
The SMT protection filter is introduced via the femoral artery with the filter membrane (140 µm filter) positioned in the aortic arch covering all three main supraaortic vessels. With the use of two nitinol frames, the device is anchored and stabilised in the aortic arch. After TAVI, the filter can either be recaptured or left in place in case of an appropriate clinical indication.
In contrast to these deflection devices, the Claret CE Pro™ filter (Claret Medical Inc., Santa Rosa, CA, USA) has features to capture and retain embolised debris from the circulation57. The Claret device is delivered via the right radial or brachial artery and, after deployment of a proximal filter basket in the innominate artery, the catheter is flexed and a distal filter basket is placed into the left carotid artery. After the procedure, particulate debris is captured within the baskets and carefully retrieved from the cerebral circulation.
Currently available protection devices still have several limitations that may affect their effectiveness:
– While the Embrella and the Claret devices do not cover the origin of the left subclavian artery and leave the left vertebral artery unprotected, the SMT protection device requires large vascular access and its stabilisation mechanism may interfere with the valve delivery system during transfemoral TAVI.
– The manipulation of the protection device catheters themselves as well as the retrieval may hypothetically result in vascular trauma or be the source of additional cerebral emboli.
Deflection rather than retention of embolised material may only shift the problem to the peripheral or renal circulation.
Another approach in the armamentarium to reduce thromboembolic events during and after TAVI is the careful consideration of antithrombotic regimens. The use of bivalirudin among patients with non-ST-segment elevation acute coronary syndromes has been shown to reduce the risk of major bleeding while maintaining a favourable outcome in terms of ischaemic events compared with the combined use of unfractionated heparin and glycoprotein IIb/IIIa inhibitors58. Furthermore, bivalirudin was found to be superior with respect to major bleeding and net adverse clinical events among patients with ST-segment elevation myocardial infarction59. As a result, bivalirudin assumes a Class IB recommendation as anticoagulant in the current ESC Guidelines on the management of acute coronary syndromes60. The pharmacological properties of this direct thrombin inhibitor have also been shown to reduce bleeding and vascular complications among patients undergoing balloon aortic valvuloplasty in the BRAVO-1 registry61. Bivalirudin as anticoagulant for patients undergoing TAVI is currently under investigation in the BRAVO-2 and 3 studies, which will provide important information on the safety and efficacy of this agent in terms of reducing the risk of bleeding and, potentially, stroke.
Although the risk of CVA is highest during the first 48 hours after TAVI, there remains a substantial risk of stroke during long-term follow-up. Optimal medical treatment aims to reduce the long-term risk-by-risk factor modification but still has to be standardised among patients undergoing TAVI. While low dose aspirin (75 mg to 100 mg qd) is recommended for prevention of thromboembolism for at least three months after surgical aortic valve replacement with bioprostheses, most observational studies of patients undergoing TAVI report on a dual antiplatelet regimen consisting of aspirin and clopidogrel (75 mg qd) for the duration of six months. Limited evidence exists on the safety and effectiveness of a single treatment after TAVI with aspirin alone62 as well as the long-term need for antiplatelet therapy. Among patients with atrial fibrillation or previous history of thromboembolism, anticoagulation with vitamin K antagonists or novel oral anticoagulants is the treatment of choice.
TREATMENT OF CEREBROVASCULAR INJURY AFTER TAVI
Analogous to the narrow treatment window for the implementation of reperfusion therapy among patients with acute myocardial infarction, the principle “time is brain” applies to the acute treatment of stroke after TAVI. In order to improve outcomes, timely assessment of the patient by a dedicated “Stroke Team” is desirable. This typically consists of neurologists with special interest in vascular disease, neuroimaging specialists, interventional neuroradiologists, neurosurgeons, and anaesthesiologists with access to specialised neurointensive care units.
In cases of acute stroke after TAVI, immediate consultation of the stroke team will initiate dedicated treatment protocols and cerebral imaging to determine the need for emergent cerebral angiography and immediate endovascular intervention. As the majority of strokes during the early periprocedural period after TAVI are due to embolisation of particulate debris or thrombus, rapid reperfusion by means of intra-arterial thrombolysis, mechanical thrombectomy or balloon angioplasty with or without stent placement may be instrumental to limit the extent of brain damage and improve recovery.
The use of intravenous thrombolysis is generally accepted for the treatment of acute stroke within a time window of three hours after symptom onset35. However, the effectiveness after periprocedural stroke with emboli of unknown origin may be limited by means of an intravenous administration. Moreover, the administration of systemic thrombolysis after TAVI with the use of large bore catheters may put patients at disproportional risk for bleeding complications related to the access site. In contrast, local intra-arterial thrombolysis has the advantage of direct injection of the thrombolytic drug into the embolus63. Using a lower dose of thrombolysis compared with systemic administration, revascularisation results and outcomes appear favourable with a combination of local application of thrombolysis and mechanical fragmentation. More recently, a dedicated EKOS MicroLysUS infusion catheter (EKOS Corp, Bothell, WA, USA) was investigated for effectiveness among patients with acute stroke64. By augmenting thrombolysis within the thrombus and creating local convection currents by using ultrasound an intense diffusion of the thrombolytic drug is achieved which may result in high rates of revascularisation within a short period of time. Whether local embolus fragmentation is effective among patients with periprocedural stroke after TAVI requires further investigation.
Endovascular interventions including mechanical thrombectomy, stent retriever thrombectomy and balloon angioplasty can be performed during endovascular treatment of acute stroke65. Stent revascularisation of the cerebral vasculature is considered an off-label use and only performed as bailout treatment, as dual antiplatelet therapy after stent implantation may favour intracranial bleeding after stroke. However, the use of removable cerebral stents to extract cerebral emboli is an alternative technique66 with high rates of flow restoration and favourable clinical outcomes with early symptom resolution.
Summary
Cerebrovascular accidents after transcatheter therapeutic interventions of the aortic valve remain an important source of concern due to the deleterious impact on prognosis and quality of life. Patients undergoing TAVI usually have multiple comorbidities and are at increased risk for periprocedural complications including stroke. Although the current incidence of CVA appears acceptable in view of the overall benefit of TAVI in this high-risk patient population, multiple efforts aim to reduce further the risk of stroke. Apart from improved implantation techniques, cerebral embolisation protection devices and novel anticoagulants with the aim of reducing the frequency of this adverse event, immediate diagnosis of stroke and rapid initiation of treatment by a dedicated stroke team are promising strategies to improve outcomes of affected patients.
Conflict of interest statement
P. Wenaweser is proctor and receives honoraria from Medtronic CoreValve and Edwards Lifesciences. F. Windecker has received honoraria and consultant fees from Edwards Lifesciences and Medtronic CoreValve. S. Stortecky has no conflict of interest to declare.

