Abstract
Guidelines on antithrombotic therapy after TAVI are scarce and no randomised evaluation has been performed to demonstrate what the best strategy is. Extrapolation from what we know on surgical heart valve replacement is also hazardous as the level of evidence is not very high and the situation, the valves and the patients are different. Patients undergoing TAVI are fragile and at high risk for both bleeding and stroke complications. In addition, the procedure itself may contribute to the occurrence of these complications rendering risk stratification a key step. Improvement in both periprocedural and post-procedural outcome is a major challenge of antithrombotic therapy. The recent release of more potent but also safer antithrombotic therapies is a unique opportunity to sort out what is the best combination for which patient. Our goal is to review the evidence and analyse what are the perspectives in this challenging area.
Introduction
The number of transcatheter aortic valve implantation (TAVI) procedures is increasing exponentially as it has been demonstrated to be a live-saving procedure. The TAVI population is frail and bears a high risk of both ischaemic stroke and major bleeds that is much higher than in conventional bioprosthetic aortic valve replacement. The 30-day rate of major bleeding and stroke exceeds 15% in this population. Both complications are independent predictors of mortality1. Guidelines on antithrombotic therapy after TAVI are scarce and no randomised evaluation has been performed to demonstrate what the best strategy is. Extrapolation from what we know on surgical heart valve replacement is also hazardous as the level of evidence is not very high and the situation, the valves and the patients are different.
Dual antiplatelet therapy (DAPT) is recommended (Class 2b, level of evidence [LOE] C)2, although more than half of senior patients display high on-clopidogrel platelet reactivity3, less than one third undergo stent implantation prior to valve replacement4 and more than one third display transient atrial fibrillation during hospital stay5. In addition, one third of patients discontinue clopidogrel prematurely and are exposed to single antiplatelet therapy. Large datasets suggest that absence of antithrombotic therapy within the first months after surgical bioprosthetic aortic valve replacement or mitral valve repair is associated with a significant increase in the risk of cardiovascular death and thromboembolic events6. Our aim is to review what the evidence on antithrombotic therapy is after TAVI.
Ischaemic events after TAVI
Ischaemic events occurring during and after TAVI procedure are mainly cerebrovascular events. The common underlying mechanism is embolisation. The pathophysiology of periprocedural and post-procedural stroke differs in many instances7,8. Periprocedural events can be triggered by the mechanical interactions between the devices (balloon valvuloplasty, delivery catheter and the bioprosthetic valves) and the native valve and can be viewed as the consequences of embolisation of calcified material or atheromatous debris from the aorta. Post-procedural events are rather triggered by the thrombogenicity of the bioprosthetic device and the modifications in blood flow rheology in addition to new onset of atrial fibrillation9. As a consequence, prevention strategies may rely on refinement in the delivery of the bioprosthetic valve, the bioprosthetic valve itself and on antithrombotic treatment using a careful risk stratification.
The rate of major stroke is as high as 3% within the first 30 days, half of which is clustered in the first 24 hours of implantation (Figure 1). However, clinical stroke (TIA and stroke) continues to be high after the first 24 hours and persists up to 60 days (more than 3%) while the vast majority of patients are on DAPT, a combination of aspirin and clopidogrel, the post-TAVI standard of care10,11. Of interest is the strong correlation between the CHA2DS2-VASc score and the risk of stroke, further highlighting the potential role of atrial fibrillation (AF) but also of the underlying vascular disease as major contributory factors12. Haemorrhagic strokes have not been quoted and should be anticipated as a key player in this fragile population.
Stroke has an established detrimental impact in the TAVI population with an at least twofold increase in mortality1.
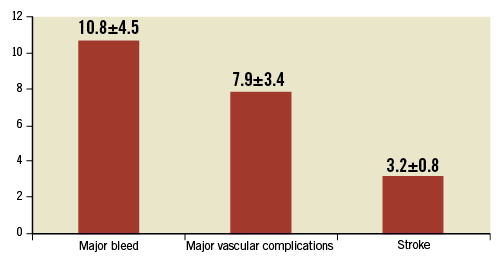
Figure 1. Rate of major events within the first 30 days following TAVI procedures as defined according to VARC in four major series9,45-47.
Bleeding events after TAVI
The rate of major bleeds according to VARC13 exceeds 10% at 30 days1. Procedural bleedings are of particular importance due to the insertion of large sheaths in fragile vascular tissues. Learning curves, decrease in sheath size and the use of percutaneous closure devices have led to a substantial reduction in these dramatic complications14,15. In-hospital life-threatening or major bleeds are a combination of mechanical and antithrombotic-driven complications that are associated with increased mortality whose magnitude is similar to that of ischaemic events (Figure 1). In particular, the role of triple antithrombotic therapy needs some investigation as it is usually administered to fragile patients.
Recommendations for antithrombotic therapy during TAVI procedures
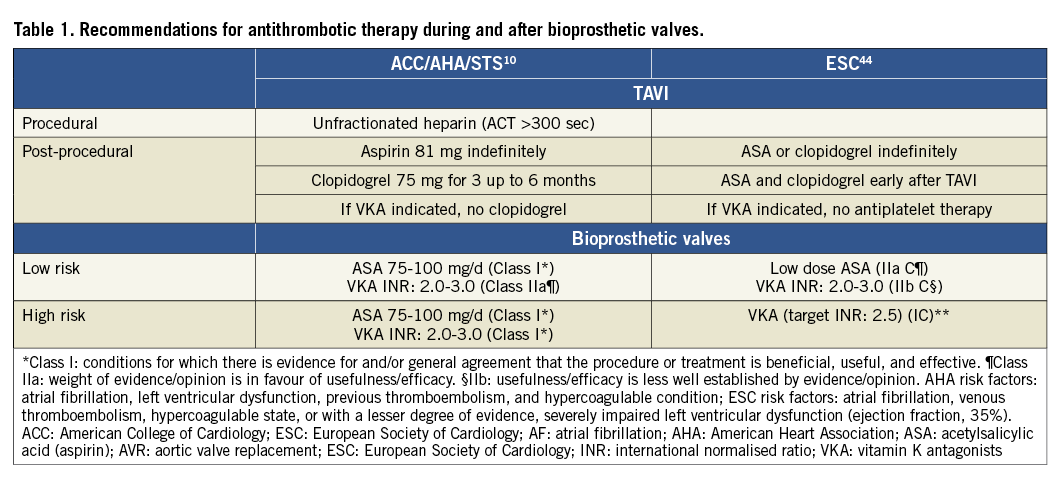
During TAVI procedures, unfractionated heparin is recommended, with a target ACT of 300 seconds or more, because of its ease of use and possible fast reversal with protamine sulphate10. An initial bolus of 5,000 units is used with additional boluses to maintain ACT above 250 seconds16,17. However, there is no evidence showing the relevance of ACT in this specific setting or for percutaneous coronary intervention (PCI) 18. Bivalirudin is under investigation in the pilot BRAVO study19, although some concerns remain when immediate reversal is required due to life-threatening vascular and bleeding complications. The safety benefit of bivalirudin was not proven in stable patients undergoing coronary stent placement when compared head to head with the recommended dose of unfractionated heparin20. In addition, TAVI procedures are characterised by the use of large sheaths with systematic closure devices and therefore a higher bleeding risk, whereby the safety benefit of bivalirudin is not known. Other possibilities should be tested with parenteral anticoagulation which has shown better safety than unfractionated heparin in PCI, e.g., intravenous enoxaparin21.
Recommendations for antithrombotic therapy after TAVI
There is currently no large dataset on how post-TAVI antithrombotic therapy is handled in real life, and on its relation to baseline clinical characteristics and to clinical outcome. The standard of care is the combination of low-dose aspirin and of a maintenance dose of 75 mg clopidogrel (Table 1). However, DAPT is recommended without clear specifications with respect to loading dose, pretreatment and duration of clopidogrel therapy10,22. In addition, the DAPT hypothesis has never been tested prospectively, and there is no robust evidence demonstrating that early thromboembolic events observed after TAVI are platelet-mediated. The ongoing Aspirin versus aspirin + clopidogRel following Transcatheter aortic valvE implantation (ARTE) pilot trial (NCT01559298) is testing the hypothesis of single antiplatelet therapy versus DAPT after TAVI in patients not requiring anticoagulation23. This is an important trial for several reasons. First, premature discontinuation of DAPT remains a concern in this elderly population but this may also apply to other antithrombotic regimens24. Second, the benefit of clopidogrel is questionable among elderly patients in whom high on-clopidogrel platelet reactivity is a common finding3. Third, the risk of bleeding is increased with DAPT25-27.
The real world
DAPT is the most widely used antithrombotic strategy after TAVI28 in more than two out of three patients, although AF is observed in more than 40% of patients. AF is common among high-risk patients with severe aortic stenosis undergoing TAVI and is associated with a greater than twofold increased risk of all-cause and cardiovascular mortality, irrespective of the type of AF12. Of importance, the gradient of risk directly correlates with the CHA2DS2VASc score, which is usually used for decision making as to whether anticoagulation should be initiated (Figure 2)29. Therefore, at least 30% of the TAVI population merits anticoagulation therapy, a strategy that is underused, especially in high CHADS risk score patients, something which has never been evaluated prospectively in post-TAVI procedures.
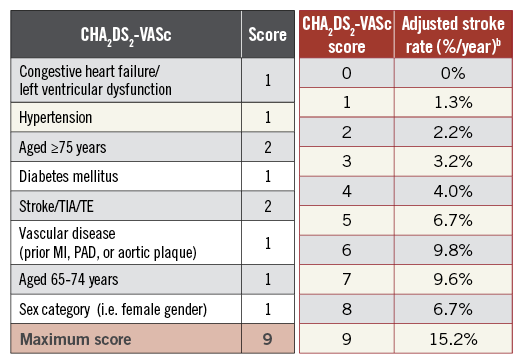
Figure 2. The CHA2DS2-VASc score with the corresponding stroke risk.
Triple therapy, a combination of vitamin K antagonists (VKA)+ aspirin+clopidogrel is used in the most gravely ill patients and is associated with an increased risk of death, stroke, embolism, or major bleeding (adjusted odds ratio, 1.78; 95% confidence interval, 1.1-2.9)28. These results are consistent with recent investigations on triple therapy following myocardial infarction or PCI in AF patients30,31. In the recent National Danish Registry, relative to DAPT, triple therapy led to a significant reduction in the rate of ischaemic stroke (HR 0.54; 95% CI: 0.28-1.01) at the cost of a significant increase in the rate of major bleeding (HR 2.67; 95% CI: 1.79-3.98), including intracranial bleeds24. This finding is very consistent in all PCI trials comparing triple versus dual antiplatelet therapy (aspirin+clopidogrel) (Figure 3)30,32-34. The combination of oral anticoagulation (OAC)+clopidogrel appears attractive and was not associated with an excess of MI or coronary death as compared to triple therapy but instead displayed a better safety30,31. Such a combination may be discussed in TAVI patients with or without prior established coronary artery disease.
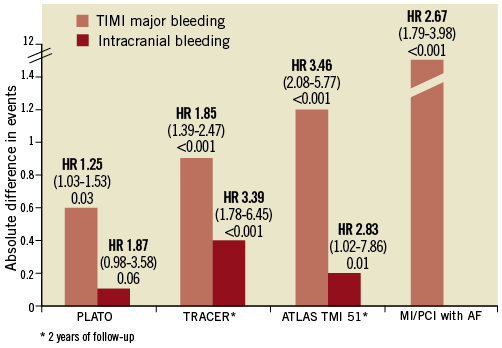
Figure 3. Major bleeding and intracranial bleeding complications associated with intensified antithrombotic regimens versus DAPT in ACS/PCI patients. Triple therapy including three antiplatelet agents (TRACER) or a combination of oral anticoagulant with DAPT is associated with a consistent increase in major bleeds.
The benefit of oral anticoagulation over DAPT in AF depends on the quality of international normalised ratio control35. It has been predicted that a time in the therapeutic range of 58% would be needed so that patients would benefit from being on OAC, a level that is rarely obtained in TAVI patients. Therefore, new strategies have to be tested versus the standard of care for which only limited evidence exists for the TAVI population.
How to stratify the risk after the TAVI procedure?
Risk stratification is a key step in the guidance of antithrombotic therapy following a TAVI procedure. The CHA2DS2-VASc score is of particular interest in this elderly population (Figure 2). This approach to risk stratification of patients with non-valvular AF defines “major (definitive)” risk factors (e.g., previous stroke/transient ischaemic attack and age ≥75 years) and “clinically relevant non-major” risk factors (e.g., heart failure, hypertension, diabetes, female gender, age 65-74 years, and atherosclerotic vascular disease)36. It has the ability to identify AF patients with no net clinical benefit or even some disadvantage from anticoagulant treatment37 but also to identify non-AF patients with a prior history of stroke and at very high risk of further cardiovascular events38. Of importance, this score was found to be associated with one-year mortality in TAVI patients. Although the vast majority of TAVI patients with AF are eligible for chronic OAC given an average CHA2DS2-VASc score ≥4 , whether non-AF patients with a CHA2DS2-VASc score ≥2 would benefit from chronic OAC is challenging.
With respect to bleeding stratification, HEMORR2HAGES appears to be the most suitable score for the TAVI population (Figure 4)39. It is a valid predictor of haemorrhage in patients who are prescribed OAC or antiplatelet therapy. In addition, it takes into account many features of frailty and could be a useful tool to trade off the risks and benefits of prescribing OAC versus antiplatelet therapy, especially among TAVI patients who are eligible for both strategies, i.e., antiplatelet or anticoagulation.
| Hepatic or renal disease | 2 |
| Ethanol abuse | 1 |
| Malignancy | 1 |
| Older (age>75) | 1 |
| Reduced platelet count or function | 1 |
| Re-bleeding risk | 1 |
| Hypertension uncontrolled | 1 |
| Genetic factors | 1 |
| Excessive fall risk or neuropsy disease | 1 |
| Stroke | 1 |
| Score | % risk/100 patients/year |
| 0 | 1.9 |
| 1 | 2.5 |
| 2 | 5.3 |
| 3 | 8.4 |
| 4 | 10.4 |
| ≥5 | 12.3 |
Figure 4. The HEMORR2HAGES score with the corresponding major bleeding risk39.
Finally, up to 35% of patients undergo coronary stenting prior to the TAVI procedure, suggesting that the risk of stent thrombosis in addition to that of AF should be taken into account in the overall risk assessment40. New P2Y12 inhibitors may be discussed when stenting is performed in the setting of acute coronary artery disease. In particular, short duration of DAPT and maintenance of prasugrel or ticagrelor without aspirin should be investigated to support further the antiplatelet hypothesis as being the best antithrombotic regimen after TAVI.
What do we need? What should be avoided?
The clinical challenge is to demonstrate that an anticoagulation strategy is superior to dual antiplatelet therapy, the standard of care. Randomisation should be stratified according to underlying AF, a major determinant of mortality. The net clinical benefit defined according to VARC definitions should be considered as the primary endpoint13, as we may anticipate a rate of bleeding events as high as the rate of ischaemic events. Post-procedural events should be differentiated from periprocedural events. The use of brain imaging should be addressed, given the multiplicity of stroke types which may have an impact both on decision making regarding antithrombotic strategy and also on the endpoint definition. Triple antithrombotic treatment should be avoided even in patients with recent stent implantation. The “What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing?” (WOEST) trial demonstrated a superior safety and a striking decrease in both stent thrombosis and all-cause mortality with a combination of warfarin and clopidogrel over triple therapy in patients with AF undergoing drug-eluting stent placement. This further raises the hypothesis as to whether a post-TAVI regimen of clopidogrel and warfarin would be a better choice rather than aspirin and warfarin. This is highlighted by the post-MI or stenting Danish registry30. Finally, the direct thrombin and factor Xa inhibitors should be considered as new perspectives in the TAVI era.
What are the perspectives?
Oral direct thrombin/Xa inhibitors have shown superiority or non-inferiority versus vitamin K antagonists to prevent cardioembolic events with a consistent reduction in intracranial bleeds in patients with non-valvular AF29,41. Apixaban is the only direct oral anti-Xa inhibitor which has demonstrated a mortality benefit and a significant reduction in major bleeds versus VKA42. Of interest, enoxaparin and rivaroxaban, anti-Xa and direct Xa inhibitors respectively, also display a benefit towards fewer coronary events after PCI for acute coronary syndrome versus placebo on top of DAPT21,34.
Antiplatelet therapy has been challenged in the arena of AF. The ACTIVE-W trial, a head-to-head comparison of vitamin K antagonists and DAPT in the setting of non-valvular AF, was prematurely stopped for superiority43. Similarly, the AVERROES trial, which has tested the direct Xa inhibitor apixaban versus aspirin in VKA non-suitable AF patients, was also prematurely stopped for superiority42. In addition and of interest, apixaban displayed a similar safety profile to aspirin.
Conclusions
The vast majority of post-TAVI ischaemic events are cerebrovascular, a major determinant of which is AF. The implanted bioprosthetic valve may add an additional prothrombotic environment magnifying the cardioembolic risk in patients who are already at high baseline risk. This further supports testing the anticoagulation and antiplatelet hypotheses in the post-TAVI setting to improve clinical outcome of these fragile patients further. New anticoagulant therapies are promising and should be compared to the standard of care, including vitamin K antagonists. Meanwhile, a careful risk stratification should be used for post-TAVI antithrombotic treatment guidance and triple therapy should be avoided and should always be short if mandatory.
Conflict of interest statement
J-P. Collet has received research grants (to the institution) from Bristol-Myers Squibb, Sanofi-Aventis, Eli Lilly, Guerbet Medical, Medtronic, Boston Scientific, Cordis, Stago, Fondation de France, INSERM, Fédération Française de Cardiologie, and Société Française de Cardiologie; and has served as a consultant to and received lecture fees from Sanofi-Aventis, Eli Lilly, and Bristol-Myers Squibb. G. Montalescot has received research grants from Abbott Vascular, AstraZeneca, Biotronik, Daiichi-Sankyo, Eli Lilly, Fédération Française de Cardiologie, Fondation de France, INSERM, Institut de France, Medtronic, Nanospheres, Pfizer, Roche, Sanofi-Aventis, Stentys, Société Française de Cardiologie, The Medicines Company, BMS, Menarini, Accumetrics; and has received consulting fees from Bayer, BMS, Boehringer-Ingelheim, Duke Institute, Europa, GSK, Iroko, Lead-Up, Novartis, Springer, TIMI group, WebMD, Wolters, AstraZeneca, Biotronik, Eli Lilly, The Medicines Company, Medtronic, Menarini, Roche, Sanofi-Aventis, Pfizer, Accumetrics.

