
Surgical bioprosthetic aortic valves were introduced with the purpose of avoiding oral anticoagulation (OAC) therapy and thereby the risk of major bleeding in elderly patients. During the last decade, transcatheter bioprosthetic aortic valves have become an alternative to surgical aortic valve replacement (SAVR), and antithrombotic therapy after transcatheter aortic valve implantation (TAVI) is mainly based on surgical practice. While waiting for the outcome of a number of ongoing trials comparing different antithrombotic regimes after TAVI, existing data indicate that, although OAC may potentially reduce the rate of ischaemic stroke as well as delay deterioration of the bioprosthetic valve, this therapy may be harmful in the elderly patient cohort currently receiving transcatheter heart valves (THV).
The TAVI procedure itself may be associated with focal vascular injury, which may include commissural tear, endothelial injury of the aortic wall, as well as exposure of calcific plaque. Furthermore, the endothelialisation of the bioprosthetic leaflets and valve frame will only be completed after months. The resulting platelet aggregation and activation of coagulation factors can lead to thrombus formation. In the setting of SAVR, endothelialisation of the bioprosthetic aortic valve is expected to be complete by three months1. The in vivo timing for endothelialisation of THV is unknown, but a high rate of embolic vascular complications has been noted in the first two months after TAVI2, corresponding well with surgical data and illustrating a role for more potent anticoagulant therapy in the initial post-procedural period.
According to current guidelines3,4, patients with no other indication for antithrombotic therapy are recommended dual antiplatelet therapy (DAPT) with aspirin and clopidogrel for three to six months followed by lifelong single antiplatelet therapy (SAPT) with aspirin (Figure 1A). However, there are differences in the most recent European and American guidelines. The ESC/EACTS guidelines suggest that SAPT may be considered instead of DAPT in case of high bleeding, whereas the AHA/ACC guidelines state that OAC with a vitamin K antagonist (VKA) to achieve an international normalised ratio (INR) of 2.5 may be reasonable for at least three months after TAVI in patients at low risk of bleeding.
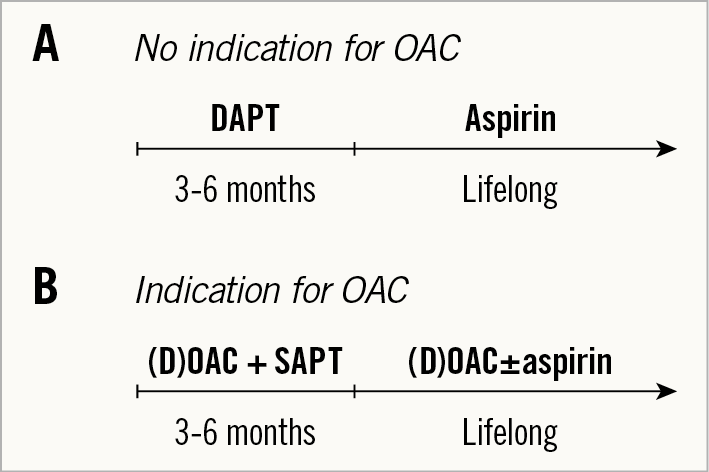
Figure 1. Current guidelines for antithrombotic therapy. A) For patients with no other indication for oral anticoagulation. B) For patients with an indication for long-term oral anticoagulants. DAPT: dual antiplatelet therapy; (D)OAC: (direct) oral anticoagulant; SAPT: single antiplatelet therapy
One argument to support the less aggressive European approach is that numerous observational studies have compared the use of DAPT versus SAPT in the initial period after TAVI with the near universal consensus being that there is no significant difference in terms of major clinical outcomes while long-term DAPT confers an elevated risk of bleeding. Similarly, in a small randomised trial between DAPT versus SAPT for three months after TAVI using the SAPIEN XT THV (Edwards Lifesciences, Irvine, CA, USA), SAPT reduced the risk for major or life-threatening bleeding, while not increasing the risk for myocardial infarction or stroke5. These results are highlighted in a series of over 16,000 TAVI patients from the STS/ACC TVT registry showing similar rates of stroke, myocardial infarction and mortality for DAPT versus SAPT, while the risk for major bleeding was significantly elevated for DAPT versus SAPT6.
On the other hand, the more aggressive antithrombotic strategy in the American guidelines is implemented in order to avoid valve thrombosis. Several studies have demonstrated the occurrence of subclinical leaflet thrombosis in both transcatheter and surgical bioprosthetic aortic valves7,8. This phenomenon is often assessed by multidetector computed tomography, which shows hypo-attenuating opacities, sometimes with reduced leaflet motion. Interestingly, it is characterised by an almost normal transvalvular gradient and no symptoms. The frequency of this finding has varied from 7% to 40%, depending on THV type as well as whether the patients receive OAC. So far, the clinical impact of subclinical leaflet thrombosis is unknown, but it has been speculated that it may progress into clinical valve thrombosis with a high transvalvular gradient, or cause stroke or early deterioration of the bioprosthetic aortic valve.
Short-term OAC is also supported by a large-scale registry study, which showed that treatment with VKA during the first three months after implantation of surgical bioprosthetic aortic valves resulted in lower rates of stroke, thromboembolic events, cardiovascular deaths, as well as bleeding incidents as compared to no VKA treatment1. However, these effects of VKA therapy did not continue after three months.
The GALILEO trial9 compared a low dose of the direct oral anticoagulant (DOAC) rivaroxaban plus an initial three months of aspirin followed by monotherapy with low-dose rivaroxaban versus DAPT for three months followed by aspirin in over 1,500 patients with successful TAVI. Although the data are still awaited, the trial was prematurely terminated during the follow-up due to numerically higher mortality or thromboembolic events, all-cause mortality, and major bleeding in the DOAC group. This indicated that, although OAC may be reasonable during the initial months after TAVI, long-term OAC may be harmful in the elderly patients who currently undergo TAVI. It could also be argued that, even if TAVI should expand to younger patients with less comorbidity in the near future, a mechanical aortic valve may be a better option if OAC is also required for THV.
For patients with an indication for OAC, e.g., due to atrial fibrillation (AFib), which is present in about one third of patients undergoing TAVI, the guidelines recommend OAC possibly together with SAPT for three to six months followed by lifelong OAC with the possibility of simultaneous aspirin (Figure 1B). Importantly, guidelines call for a reluctance for triple therapy.
In consideration of the above-mentioned elevated bleeding risk among patients undergoing TAVI, long-term OAC may not be attractive in all patients with AFib. A short course of post-procedural OAC may be acceptable; however, left atrial appendage occlusion (LAAO) has emerged as a reasonable alternative for thromboembolic prophylaxis in this patient group. In a small study, patients with AFib were treated with either DOAC or LAAO following TAVI and showed no difference in outcomes with regard to stroke, but a significant reduction in major bleeding events in patients who received LAAO10.
Considering the current state of evidence, what might an optimal antithrombotic regimen after TAVI look like? It is reasonable to consider abandoning the current use of DAPT given recent trial data. The introduction of a short course of OAC in the initial post-procedural risk period followed by long-term aspirin may potentially have advantages in terms of early thrombotic risk and valve haemodynamics (Figure 2A). For patients with AFib and indication for long-term OAC, short-term OAC during the phase of endothelialisation of the THV is probably required. After this period, LAAO is an attractive option in patients at high bleeding risk (Figure 2B).
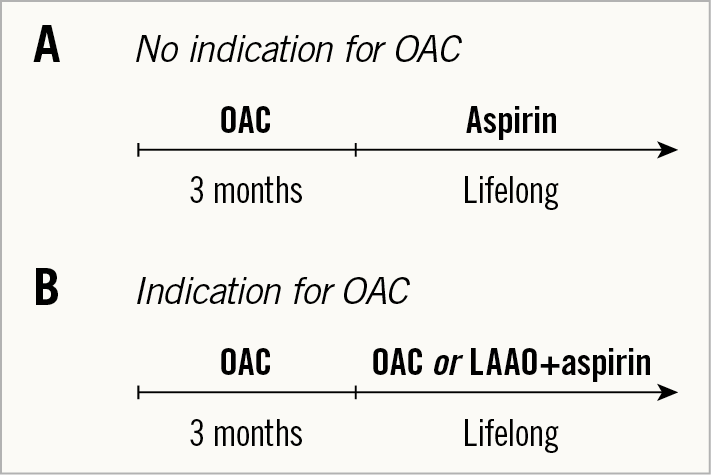
Figure 2. Proposed antithrombotic therapy. A) For patients with no other indication for oral anticoagulation. B) For patients with an indication for long-term anticoagulants. DAPT: dual antiplatelet therapy; (D)OAC: (direct) oral anticoagulant; LAAO: left atrial appendage occlusion; SAPT: single antiplatelet therapy
Selection of the antithrombotic strategy after TAVI remains in question; however, this is an expanding field of research. The questions become even more complex when treating patients with other comorbidities, e.g., ischaemic heart disease, peripheral vascular disease, chronic kidney disease, as well as extending TAVI to patients of a lower age and with fewer comorbidities.
Acknowledgements
B. Wilkins is supported by a research grant from the Heart Foundation of New Zealand (grant #1778).
Conflict of interest statement
L. Søndergaard has received consultant fees and institutional research grants from Abbott, Bayer, Boston Scientific, Edwards Lifesciences, Medtronic, and Symetis. The other authors have no conflicts of interest to declare.

