
Abstract
Aims: The safety and efficacy of single vs. dual antiplatelet therapy (DAPT) in patients undergoing TAVI remain to be addressed. The aim of our study was to evaluate the usefulness of a DAPT compared to a single platelet therapy in patients undergoing TAVI with a balloon-expandable prosthesis.
Methods and results: All consecutive patients enrolled in the ITER registry were included. Patients undergoing TAVI discharged with aspirin alone were compared to those taking DAPT before and after selection using propensity score with matching. Subgroup analysis was performed for those on OAT. Prosthetic heart valve dysfunction at follow-up was the primary endpoint, whereas all-cause death, cardiovascular death, bleedings, vascular complications and cerebrovascular accidents were the secondary ones. From 1,364 patients, after propensity score with matching, 605 were selected for each group (aspirin alone vs. DAPT). At 30 days, rates of VARC mortality were lower in patients with aspirin alone (1.5% vs. 4.1%, p=0.003), mainly driven by a reduction of major vascular complications (5.3% vs. 10.7%, p<0.001) and of major bleedings (6.6% vs. 11.5%, p<0.001), without a difference in prosthetic heart valve dysfunction after 45±14 months (2.8% vs. 3.0%, p=0.50). These results were confirmed on multivariable analysis.
Conclusions: After TAVI with a balloon-expandable prosthesis, aspirin alone does not increase the risk of prosthetic valve dysfunction, and reduces the risk of periprocedural complications and of 30-day all-cause death.
Abbreviations
ASA: aspirin
CAD: coronary artery disease
DAPT: dual antiplatelet therapy
IQR: interquartile range
OAT: oral anticoagulation
PS: propensity score
RCT: randomised controlled trial(s)
TAVI: transcatheter aortic valve implantation
TIA: transient ischaemic attack
VARC: Valve Academic Research Consortium
Introduction
Aortic valve replacement is the first-line therapy for patients with symptomatic aortic valve stenosis1-4. In recent years, transcatheter aortic valve implantation (TAVI) has emerged as the first choice for high-risk patients not suitable for a conventional aortic valve replacement5-10. The optimal peri-TAVI management of antithrombotic therapy represents one of the most relevant issues for these patients. Current guidelines recommend dual antiplatelet therapy with low-dose aspirin (ASA) and clopidogrel 75 mg/day for the first six months, followed by ASA lifelong11,12. Unfortunately, the strength of this recommendation is weak and mainly based on expert consensus; heterogeneity among centres in the management of antiplatelet therapy is present. Actually, due to their high-risk features, these patients are frequently exposed to periprocedural bleedings, with an ominous impact on prognosis13. At the same time, most of the indications for an antiplatelet/anticoagulation regimen after surgical biological valve implantation have come from consensus conference mainly based on investigational data from patients with mechanical valves14,15.
Regarding TAVI, a recent meta-analysis from Hassel et al16 on about 600 patients showed a trend towards less life-threatening and major bleeding without an increase of thromboembolic events, although this was limited by the small sample size of patients.
Consequently, we analysed the data from ITER17, an “all-comers”, real-world, independent, multicentre registry including 1,656 patients treated with a balloon-expandable prosthesis, to identify the optimal management of antiplatelet therapy, evaluating the safety and efficacy of single vs. dual antiplatelet therapy (DAPT). The working hypothesis of our study was that the increase in bleeding and vascular access complications observed with DAPT exceeds the potential reduction of prosthetic heart valve dysfunction and TIA/stroke which may be obtained with aspirin and clopidogrel.
Finally, we also performed a dedicated analysis of patients with an indication for oral anticoagulation therapy (OAT).
Methods
The ITER registry includes 33 Italian centres performing balloon-expandable TAVI, taking into account virtually the entire national experience. Our study, based on the ITER registry, is a retrospective analysis of prospectively collected data. All patients undergoing balloon-expandable TAVI at each centre between 2007 and 2012 were enrolled in this registry. Ethics committees approved data collection and patient informed consent was always collected. As this is a real-world all-comers experience, patient selection and procedure strategy were carried out according to the policies, experience and protocols of the individual sites. All procedures were performed using Edwards SAPIEN and SAPIEN XT prostheses (Edwards Lifesciences, Irvine, CA, USA) with a retrograde or antegrade approach. Patients were stratified according to therapy at discharge:
– Aspirin alone vs. aspirin and clopidogrel for at least six months.
– Aspirin and OAT with vitamin K antagonists vs. OAT with vitamin K antagonists alone.
DATA AND DEFINITIONS
Preoperative risk factors were defined according to the EuroSCORE (ES) classification. Presence of preoperative coronary artery disease was considered according to STS score definitions. Postoperative outcomes were defined according to the updated Valve Academic Research Consortium (VARC-2) definitions18.
ENDPOINTS
Prosthetic heart valve dysfunction at follow-up (defined as diagnosis of an aortic valve less than 1.2 cm², an increase of medium gradient of more than 20 mmHg and a peak velocity of more than 3 m/sec, excluding aortic valve regurgitation) was the primary endpoint, while all-cause death, cardiovascular death, bleedings, vascular complications and cerebrovascular accidents at 30 days and at follow-up were the secondary ones19. All the endpoints were adjudicated according to VARC-2 definitions18. Major bleedings at follow-up were defined as those needing hospitalisation and/or transfusion.
FOLLOW-UP
Patients underwent clinical and echocardiographic assessment at the study site before the operation, at hospital discharge, and then according to each centre’s protocol. Follow-up time points were requested at three to six months, at one year and every year thereafter.
STATISTICAL ANALYSIS
Continuous variables are presented as means±standard deviation or median with the interquartile range (IQR). Categorical variables are presented as frequency (%). Categorical variables were compared with Fisher’s exact test. Parametric distribution of continuous variables was tested graphically and with the Kolmogorov-Smirnov test, and the appropriate analyses were used in accordance with the results. Multiple imputation using a bootstrapping-based expectation-maximisation algorithm was used to obtain three imputed data sets (http://www.jstatsoft.org/v45/i07/). Rubin’s method was used to combine the results from each of the imputed data sets (Amelia R package). Due to lack of randomisation with regard to DAPT administration following TAVI, a propensity score (PS) was generated for each patient from a multivariable logistic regression model based on pre-treatment covariates as independent variables, with DAPT versus aspirin only administration as a binary dependent variable. Pairs of patients were derived using greedy 1:1 matching with a calliper width of 0.2 standard deviation of the logit of the PS (http://CRAN.Rproject.org/package=nonrandom)20. The quality of the match was assessed by comparing selected pre-treatment variables in propensity score-matched patients using the standardised mean difference (SMD), for which an absolute standardised difference of greater than 20% is suggested to represent meaningful covariate imbalance. A Cox regression model, stratified on the matched pairs and adjusted for other medications at discharge, was used to estimate the treatment effect (i.e., DAPT vs. aspirin only) on outcomes of interest. This approach accounts for the within-pair homogeneity by allowing the baseline hazard function to vary across matched sets (http://CRAN.R-project.org/package=survival). The Schoenfeld residuals test was used to test the independence between residuals and time and hence to test the proportional hazards assumption in Cox models (all p-values were >0.05). All p-values <0.05 were considered to indicate statistical significance. All statistical analyses were performed using R Statistical Software, version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Moreover, logistic regression was performed to assess the impact of therapy on in-hospital major bleedings, while Cox multivariate analysis was performed for valve dysfunction. All statistical analyses were performed with SPSS Version 21 (IBM Corp., Armonk, NY, USA), and differences were considered significant at α=0.05.
Results
TOTAL STUDY COHORT
One thousand six hundred and fifty-six (1,656) patients were included, being 1,364 not on OAT (605 on aspirin and 759 on dual antiplatelet therapy), and 292 on OAT (131 on OAT and 161 on OAT and aspirin).
TOTAL STUDY COHORT WITHOUT PATIENTS ON OAT
Six hundred and five patients treated with aspirin and 759 with DAPT were enrolled (Figure 1). Baseline clinical risk factors differed significantly between the groups. Patients treated with DAPT were less frequently female (64% vs. 58%, p=0.03), with higher rates of hypertension (77% vs. 84%, p=0.02), of previous myocardial infarction (13% vs. 18%, p=0.03), of peripheral artery disease (33% vs. 37% p=0.04), of neurological dysfunction (7% vs. 10% p=0.04), and of atrial fibrillation (10% vs. 17%, p<0.001). DAPT patients were more likely to be treated with valvuloplasty before TAVI (7% vs. 12%, p=0.03), and with transfemoral access (63% vs. 74%, p<0.001).
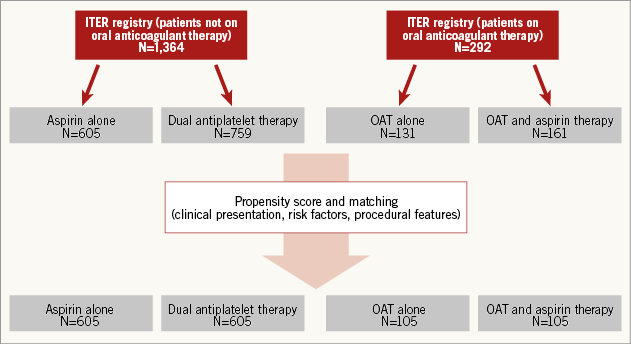
Figure 1. Selection for propensity score and matching analysis between patients on and not on OAT.
At 30 days, all-cause mortality was lower in patients treated with aspirin alone (1.5% vs. 4.1%, p=0.003), mainly due to reduced risk of major vascular complications (5.3% vs. 10.7%, p<0.001), and of major bleedings (6.6% vs. 11.5%, p<0.001), and without increased risk of stroke (1.5% vs. 1.3%, p=0.59).
After a median follow-up of 45.0±14 months, prosthetic valve dysfunction did not differ (2.8% vs. 3.0%, p=0.47), likewise all-cause mortality (26% vs. 27%, 0.48) and the risk of stroke and transient ischaemic attack (TIA) (0.7% vs. 1.6%, p=0.92), although with higher risk of major bleedings in the group of patients treated with DAPT (1.2% vs. 3.6%, p<0.001).
MATCHED STUDY COHORT WITHOUT PATIENTS ON OAT AFTER PROPENSITY SCORE ANALYSIS
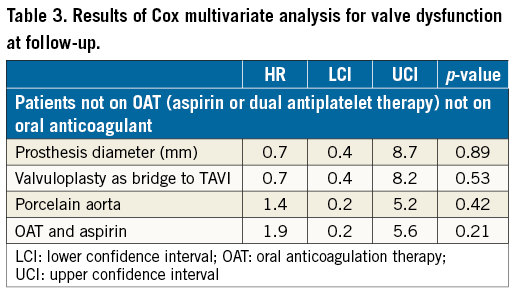
After propensity score with matching analysis, 605 patients treated with aspirin and 605 treated with DAPT with similar clinical presentation, baseline risk factors and angiographic features were selected (Figure 1, Table 1). Therefore, the results of the initial study on the overall population were reproducible. In fact, at 30-day follow-up, rates of death were higher in the DAPT group (1.5% vs. 4.5%, p 0.002), due to a higher risk of major and minor vascular complications (respectively, 5.3% vs. 12.3% and 4.6% vs. 7.9%, p<0.001) and of major and minor bleedings (respectively, 6.7% vs. 12.3% and 2.0% vs. 7.3%, p<0.001). Risk of life-threatening bleedings and of stroke did not differ between groups (respectively, 7.6% vs. 9.8%, p=0.65, and 1.6% vs. 1.8%, p=0.58) (Figure 2A).
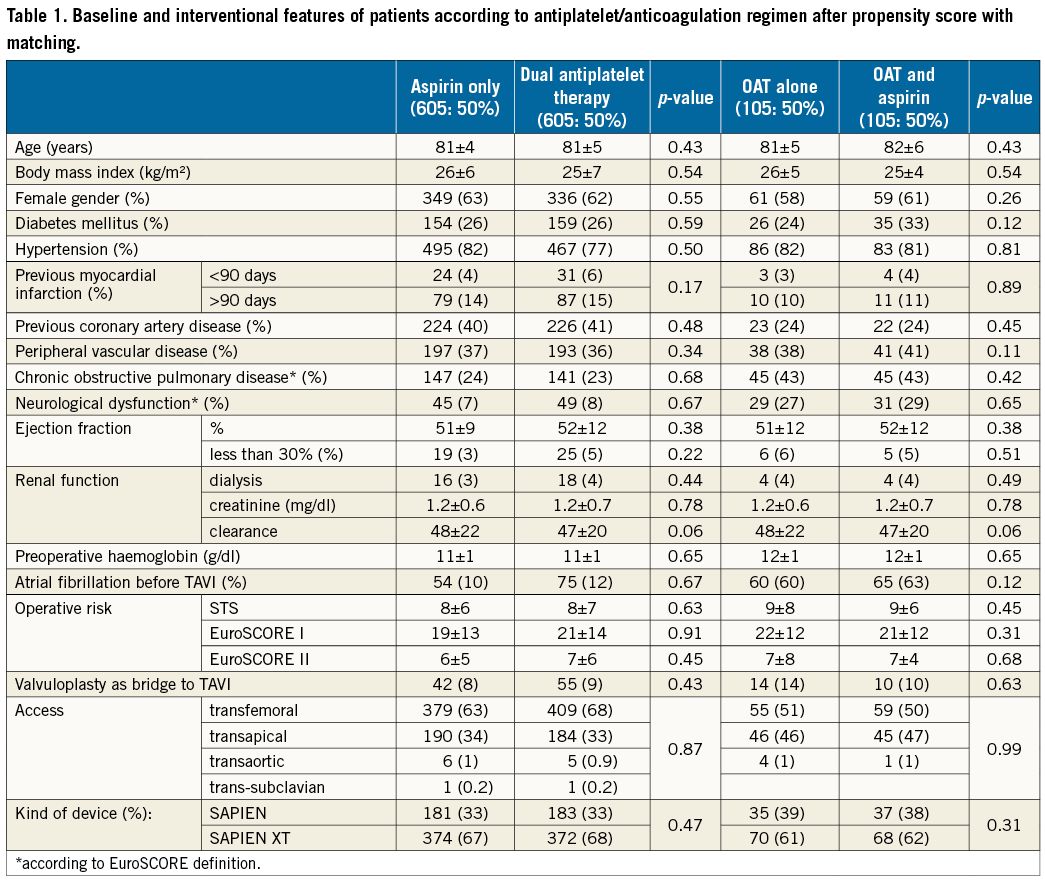
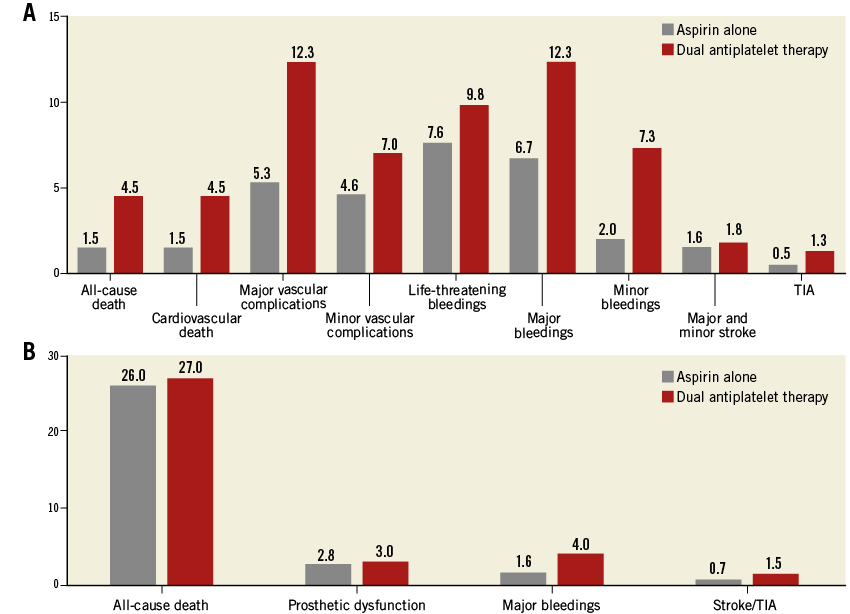
Figure 2. Patients not on anticoagulant therapy. A) Thirty-day and B) long-term outcomes after propensity score and matching analysis.
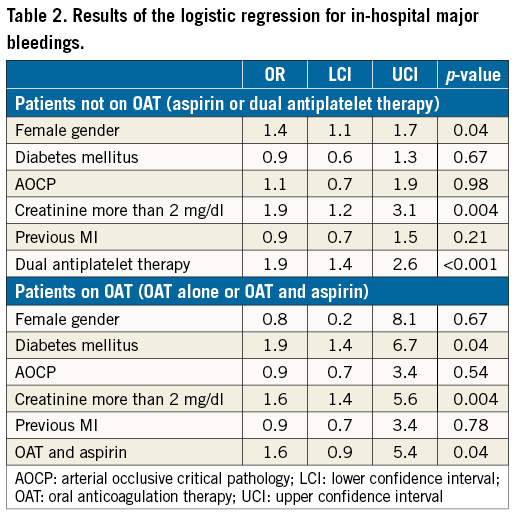
At follow-up, the risk of prosthetic heart dysfunction did not differ between the two groups (2.8% vs. 3.0%, p=0.50), nor did that of all-cause death (26% vs. 27%, p=0.69) or of stroke/TIA (0.7% vs. 1.5%, p=0.13), while patients on DAPT were more likely to experience major bleedings (1.6% vs. 4.0%, p<0.001) (Figure 2B). The logistic regression and the Cox multivariate analysis confirmed the present results (Table 2, Table 3).
TOTAL STUDY COHORT WITH PATIENTS ON OAT
One hundred and thirty-one patients treated with OAT and 161 with OAT and aspirin were enrolled (Figure 1).
Patients discharged with aspirin and OAT were less frequently female (64% vs. 54%, p=0.07), presented with higher rates of coronary artery disease (25% vs. 39%, p=0.01) and were treated more frequently with the transfemoral approach (41% vs. 66%, p<0.001).
For 30-day outcomes, all-cause mortality did not differ (3.8% vs. 3.4%, p=0.61), despite higher risk of minor vascular complications (4% vs. 11%, p=0.04) and of life-threatening bleedings in patients treated with OAT plus aspirin (4% vs. 9%, p=0.03).
After a median follow-up of 45.0±14 months, similar rates of prosthetic valve dysfunction (2.0% vs. 2.0%, p=0.56), of all-cause mortality (37% vs. 32%, p=0.22) and of stroke and TIA (4.0% vs. 3.0%, p=0.45) were reported, but with increased risk of major bleedings in patients treated with OAT and aspirin (2% vs. 4%, p<0.0).
MATCHED STUDY COHORT FOR PATIENTS WITH OAT AFTER PROPENSITY SCORE ANALYSIS
After propensity score with matching analysis, 105 patients from each group were included, with well-balanced baseline and procedural features (Table 1). At 30-day follow-up, rates of all-cause death were lower in the OAT alone group, despite being not significant (2.9% vs. 5.7%, p=0.44), with a higher risk of life-threatening bleedings and minor vascular complications with OAT and aspirin (Figure 3A).
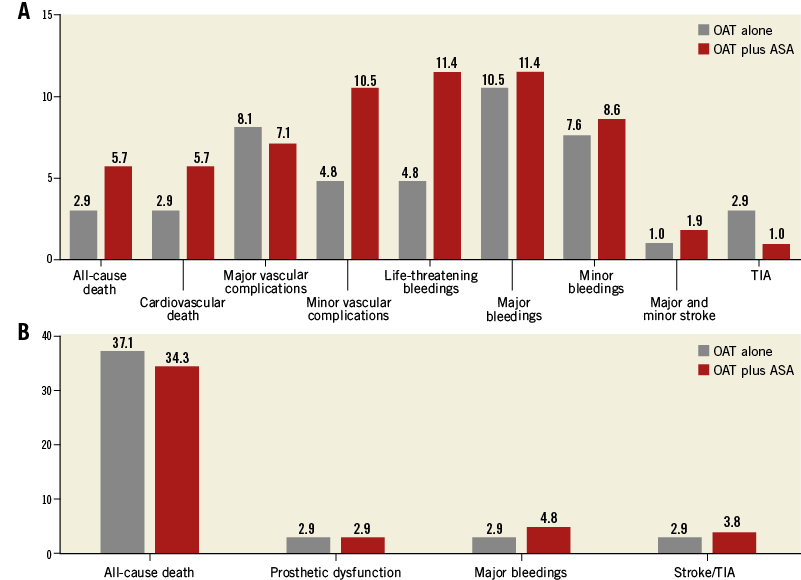
Figure 3. Patients on anticoagulant therapy. A) Thirty-day and B) long-term outcomes after propensity score and matching analysis.
At follow-up, the risk of prosthetic heart dysfunction did not differ between the two groups (2.9% vs. 2.9%, p=0.65), with a higher but not significant risk of major bleedings for patients treated with OAT plus aspirin (2.9% vs. 4.8%, p=0.36) and a similar rate of stroke/TIA (2.9% vs. 3.8%, p=0.50) (Figure 3B). The logistic regression and the Cox multivariate analysis confirmed the present results (Table 2).
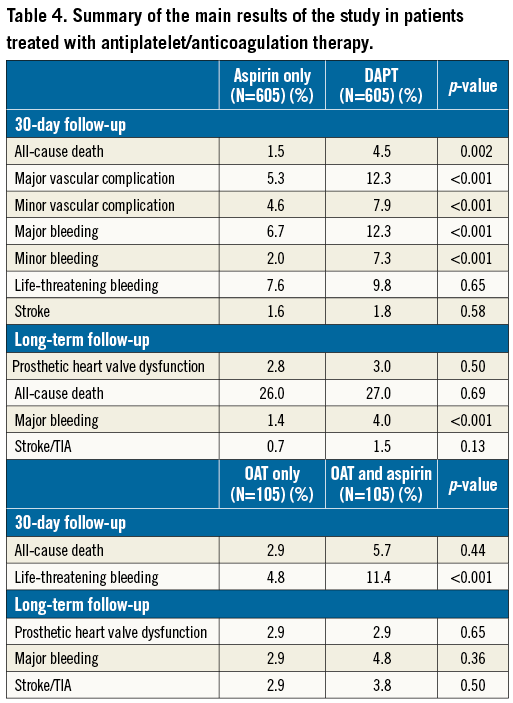
Finally, a summary of the main findings of our study is presented in Table 4.
Discussion
The main findings of our work are: 1) aspirin monotherapy does not increase the risk of prosthetic valve dysfunction, and reduces the risk of 30-day major vascular complications and bleedings which were confirmed to be independent predictors of 30-day all-cause mortality; 2) aspirin monotherapy reduces the rate of 30-day mortality; and 3) patients treated with OAT plus aspirin show a similar risk of valve dysfunction, with higher risk of bleedings at 30 days compared to patients treated with OAT alone.
The optimal management of antiplatelet therapy in patients who have undergone TAVI is still a matter of debate16,21-23. Only two small randomised controlled trials (RCT) have compared aspirin monotherapy with DAPT for three to six months after TAVI. They both found no difference in terms of safety and efficacy between the two study arms but a significant increase in the incidence of major bleedings in patients treated with DAPT21,22. These data were recently endorsed by a meta-analysis from Hassel et al on about 600 patients that showed a trend towards less life-threatening and major bleeding without an increase of ACS, strokes and mortality in patients treated with aspirin monotherapy16, as did a recent prospective study performed on about 500 patients23. Our study performed on more than 1,000 patients confirms these findings and shows that late valve dysfunction, all-cause mortality and stroke are unrelated to the antiplatelet therapy, as suggested by previous European registries24,25.
Patients treated with DAPT peri-TAVI are at increased risk of major bleeding. Often DAPT is mandatory because patients are affected by concomitant coronary artery disease (CAD)26, but the increased risk of bleeding is present also in patients without CAD. The patients who undergo TAVI are usually elderly, with many comorbidities, a low ejection fraction and with higher incidence of chronic kidney disease. All of these factors contribute to an increase in the risk of bleeding27. Our study, showing comparable efficacy endpoints and lower incidence of bleedings in patients treated only with ASA, suggests re-evaluating the guideline recommendations on antiplatelet therapy, at least in those patients without compelling indications to DAPT such as CAD with a recent revascularisation. This statement is reinforced by the finding of increased 30-day mortality in patients treated with DAPT. Such mortality is reduced in those who were treated with aspirin alone and is mainly attributable to the increased incidence of major bleeding. The latter is responsible for an increase in deaths, especially in frail patients such as those who undergo TAVI13,28.
The original idea of using DAPT therapy in patients undergoing biological valve implantation stems from three practical problems: 1) the high incidence of thrombocytopaenia observed after the first procedures that were performed with extracorporeal circulation29, 2) the time needed for prosthesis endothelialisation, and 3) the need to reduce the incidence of thromboembolism. The advances in valve design and technical improvement in deployment no longer require the use of extracorporeal circulation, removing one potential source of platelet activation30. Moreover, the SAPIEN® and SAPIEN XT® are biological prosthetic valves made of bovine pericardial tissue and, despite the fact that complete endothelialisation is probably reached in about three months31, their thrombogenicity seems to be very low, as already demonstrated by a large European registry24. The ITER registry confirms this datum and does not show a difference in thrombotic events between patients treated with ASA and those treated with DAPT. This further weakens the evidence in favour of DAPT in patients undergoing TAVI.
Limitations
The present study has many limitations. First, it has a retrospective design, despite being performed in high-volume centres and with high quality both for TAVI and for research. Second, the propensity score may allow adjustment of known confounders, but not for those not recorded, differently from RCTs. Third, the primary endpoint is an infrequent event, and consequently, despite well balanced results from propensity score (Supplementary Appendix, Supplementary Tables 1-4) the present analysis may be underpowered. Moreover, given the observational design, no sample size calculation was performed. Fourth, in our analysis we considered only two models of balloon-expandable valves; both of them are no longer used. However, our analysis is focused on the efficacy and safety of the use of a single antiplatelet therapy and, despite our results being not definitive because they are derived from retrospective data, we can assume that they can apply to new models of balloon-expandable biological prosthetic valves. Moreover, no data about the loading of clopidogrel were reported.
Finally, our results are mainly driven by the higher incidence of bleeding and vascular complications in the DAPT groups. Despite the fact that the use of smaller sheaths has significantly reduced the incidence of vascular complications, these are still the most common complications and have the most impact on prognosis in patients undergoing TAVI13,28. The use of a single antiplatelet agent could lead to a reduction of major bleeding from the access site, as suggested from our study.
Conclusions
After TAVI with a balloon-expandable prosthesis, aspirin alone seems not to increase the risk of prosthetic valve dysfunction, and seems to reduce the risk of periprocedural complications and of 30-day all-cause death. For patients needing OAT, oral anticoagulation alone reduces periprocedural complications with a similar safety profile to a combination with aspirin at follow-up. Our findings should be confirmed by a large randomised controlled trial before they can be recommended in everyday practice.
| Impact on daily practice Patients after TAVI are treated with DAPT for three or six months. This practice is mainly based on expert consensus. DAPT is affected by a higher number of bleedings if compared to single antiplatelet therapy. The results of our study show a lower incidence of bleeding without an increase in valvular dysfunction and procedural complications. If further studies with an RCT design confirm these data, peri-TAVI antiplatelet therapy management could change in favour of mono antiplatelet therapy. |
Appendix. ITER Investigators
Alaide Chieffo, Ospedale San Raffaele, Milan, Italy; Gennaro Giustino, Ospedale San Raffaele, Milan, Italy; Tommaso Regesta, Divisione di Cardiochirurgia, IRCCS San Martino-IST, Genoa, Italy; Massimo Napodano, Department of Cardiac, Thoracic and Vascular Sciences - University of Padua, Padua, Italy; Davide Gabbieri, Hesperia Hospital, Modena, Italy; Francesco Saia, AOU di Bologna, Policlinico S. Orsola-Malpighi, Bologna, Italy; Corrado Tamburino, Ospedale Ferrarotto, Università di Catania, Catania, Italy; Diego Cugola, AO Papa Giovanni XXIII, Bergamo, Italy; Marco Aiello, IRCCS Policlinico S. Matteo, Pavia, Italy; Francesco Sanna, AO Brotzu, Cagliari, Italy; Alessandro Iadanza, AOU Policlinico Le Scotte, Siena, Italy; Esmeralda Pompei, AOUD Santa Maria della Misericordia, Udine, Italy; Pierluigi Stefàno, AOU Careggi, Florence, Italy; Antioco Cappai, Humanitas Research Hospital, Rozzano, Italy; Alessandro Minati, Ospedale Cattinara, Trieste, Italy; Mauro Cassese, Clinica S. Maria, Bari, Italy; Gian Luca Martinelli, Clinica S. Maria, Bari, Italy; Andrea Agostinelli, Ospedale Maggiore, Parma, Italy; Rosario Fiorilli, AO San Camillo Forlanini, Rome, Italy; Francesco Casilli, IRCCS Policlinico San Donato, Milan, Italy; Maurizio Reale, A.O. Cesare Biagio e Arrigo, Alessandria, Italy; Francesco Bedogni, Istituto Clinico Sant’Ambrogio, Milan, Italy; Anna Sonia Petronio, AOU Pisana, Pisa, Italy; Pierluigi Omedè, Dipartimento di Scienze Mediche, Divisione di Cardiologia, Città della Salute e della Scienza, Turin, Italy; Antonio Montefusco, Dipartimento di Scienze Mediche, Divisione di Cardiologia, Città della Salute e della Scienza, Turin, Italy; Rosa Alba Mozzillo, AOU Federico II, Naples, Italy; Roberto Bonmassari, Ospedale S. Chiara, Trento, Italy; Carlo Briguori, Clinica Mediterranea, Naples, Italy; Armando Liso, Città di Lecce Hospital, Lecce, Italy; Gennaro Sardella, Policlinico Umberto I, Rome, Italy; Giuseppe Bruschi, Ospedale Niguarda, Milan, Italy; Gino Gerosa, Department of Cardiac, Thoracic and Vascular Sciences - University of Padua, Padua, Italy; Mauro Rinaldi, Dipartimento di Scienze Mediche, Divisione di Cardiologia, Città della Salute e della Scienza, Turin, Italy; Francesco Romeo, Università degli Studi Tor Vergata, Rome, Italy.
Conflict of interest statement
F. D’Ascenzo was supported by a Società Italiana di Cardiologia (SIC) grant. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. List of participating centres.
Supplementary Table 1. Standardised difference for baseline features of patients according to antiplatelet regimen before and after propensity score with matching.
Supplementary Table 2. Standardised difference for interventional features of patients according to antiplatelet regimen before and after propensity score with matching.
Supplementary Table 3. Standardised difference for baseline features of patients according to antiplatelet/anticoagulation regimen before propensity score with matching.
Supplementary Table 4. Standardised difference for interventional features of patients according to antiplatelet/anticoagulation regimen before propensity score with matching.

