Abstract
The clinical and demographic characteristics of patients undergoing TAVI pose unique challenges for developing and implementing optimal antithrombotic therapy. Ischaemic and bleeding events in the periprocedural period and months after TAVI still remain a relevant concern to be faced with optimised antithrombotic therapy. Moreover, the antiplatelet and anticoagulant pharmacopeia has evolved significantly in recent years with new drugs and multiple possible combinations. Dual antiplatelet therapy (DAPT) is currently recommended after TAVI with oral anticoagulation (OAC) restricted for specific indications. However, atrial fibrillation (which is often clinically silent and unrecognised) is common after the procedure and embolic material often thrombin-rich. Recent evidence has therefore questioned this approach, suggesting that DAPT may be futile compared with aspirin alone and that OAC could be a relevant alternative. Future randomised and appropriately powered trials comparing different regimens of antithrombotic therapy, including new antiplatelet and anticoagulant agents, are warranted to increase the available evidence on this topic and create appropriate recommendations for this frail population. Meanwhile, it remains rational to adhere to current guidelines, with routine DAPT and recourse to OAC when specifically indicated, whilst always tailoring therapy on the basis of individual bleeding and thromboembolic risk.
Pathophysiology
Calcific aortic stenosis (AS) is the most frequent manifestation of valvular heart disease in the elderly, and its prevalence continues to grow as our population ages. Transcatheter aortic valve implantation (TAVI) has become the therapy of choice for patients with severe AS who are deemed to be inoperable or at high risk for conventional surgical aortic valve replacement (SAVR). The application of TAVI in patients at low to intermediate risk is currently being investigated and it is therefore feasible that the procedure will be offered to an increasing number of patients in the near future.
Like all other vascular interventional or surgical procedures, TAVI carries thrombotic stroke, myocardial infarction or systemic embolism and periprocedural bleeding risks. Importantly, the thrombotic risk also extends during follow-up, particularly in the presence of atrial fibrillation (AF).
The risk of stroke is highest in the periprocedural period owing to the mechanics of valve positioning and implantation1. Indeed, stenotic aortic valves, unlike normal aortic valve leaflets, are characterised by large amounts of tissue factor and thrombin that increase inflammation and thrombogenicity. Unlike SAVR, the diseased native valve remains in situ during (and after) TAVI and may be mechanically damaged, leading to the exposure and/or embolism of valvular components into the arterial circulation. Additionally, insertion of a prosthesis without removal of the diseased aortic valve creates an irregular zone around the valve frame with modified flow patterns that may predispose to thrombus formation, particularly in the case of small valve sizes with associated patient–prosthesis mismatch. It has been demonstrated that cerebral emboli associated with TAVI can be composed of thrombotic or calcific atherosclerotic material. It remains unclear whether the stroke potential of these two subtypes of embolic material is alike. Importantly, TAVI patients remain at risk of stroke throughout the first months after the procedure. In these patients, mechanisms other than valve manipulation seem to be involved, such as aortic wall injury, post-traumatic surface exposure with consequent activation of the haemostatic system, turbulence or local blood stasis. In addition to the prothrombotic environment related to valve implantation and procedure-related aortic damage, roughly one third of TAVI patients have pre-existing AF and a further variable percentage (ranging from 1-30%) experience new-onset post-procedural AF, which is known to increase the risk of thrombotic complications further2.
The choice of antithrombotic therapy
Against this background, establishing the optimal antithrombotic therapy for TAVI patients remains a challenge, largely due to the lack of properly powered studies to inform practice. Unfractionated heparin (UFH) is the most common method of anticoagulation during the procedure. In the PARTNER study, UFH was administered as a parenteral bolus of 5,000 IU followed by additional doses to achieve an activated clotting time (ACT) ≥250 s. A subsequent American consensus document recommended a target ACT ≥300 s with reversal of UFH following the procedure with protamine sulphate at a milligram-to-milligram neutralisation dose. The role of bivalirudin instead of UFH remains unclear and is currently being investigated in the ongoing multicentre open-label pilot study BRAVO 2/3 (Effect of Bivalirudin on Aortic Valve Intervention Outcomes 2/3), randomising 870 patients to bivalirudin or UFH (NCT01651780).
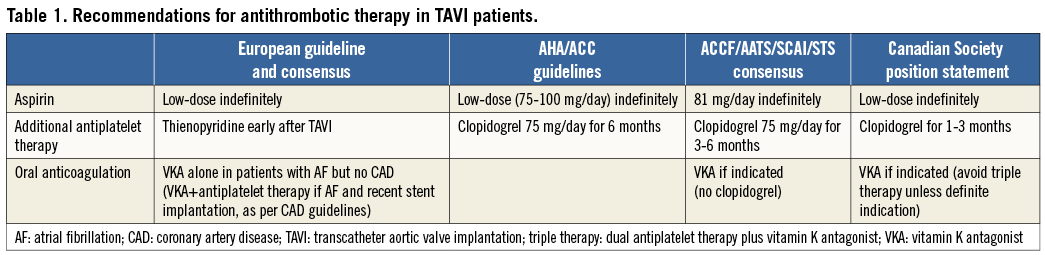
Adequately powered studies addressing the optimal antithrombotic therapy after TAVI are non-existent. It may be reasonable to speculate that TAVI patients may benefit from similar antithrombotic treatment as currently used after SAVR with a biological prosthesis. However, it is relevant to emphasise that percutaneous valves have leaflets composed of biological material and a metallic frame similar to vascular stents. Moreover, there is little evidence to demonstrate the ideal antithrombotic regimen after SAVR with biological prostheses and no uniformity amongst guidelines: a) European guidelines support use of aspirin (IIa recommendation) or vitamin K antagonists (VKA, IIb recommendation) for three months; b) AHA/ACC guidelines recommend long-term low-dose aspirin (IIa recommendation; level of evidence [LOE] B) while VKA are considered reasonable for the first three months (IIb recommendation, LOE C); c) ACCP guidelines support low-dose aspirin over VKA (Grade 2C). Therefore, some but not all guidelines recommend VKA in the first 3-6 months after SAVR whereas aspirin may be a preferred long-term treatment.
In TAVI patients, secondary prevention regimes based on antiplatelet therapy have been the most widely accepted treatment option. Given the increased thrombotic risks related to TAVI valve structure, dual antiplatelet therapy (DAPT) with aspirin (indefinitely) and clopidogrel (3-6 months) –in the absence of an indication for anticoagulation– is a widely accepted empirical strategy which has been incorporated into practice guidelines (Table 1). DAPT is also important for the many TAVI patients with concomitant coronary artery disease (CAD) who undergo stenting.
Nevertheless, the benefits of DAPT have been questioned, and recent observations that the addition of clopidogrel to aspirin does not seem to improve efficacy and safety may trigger a paradigm shift in the choice of optimal antithrombotic therapy after TAVI. Pooled analysis of individual patient data from 672 participants comparing aspirin alone versus DAPT after TAVI showed no difference in the rate of 30-day net adverse clinical and cerebral events, but a trend towards less life-threatening and major bleeding was observed in favour of aspirin alone3. Conversely, it should be considered that: a) only four studies based on small numbers of patients and events are available and in only two of them was treatment randomly allocated; b) high on-treatment platelet reactivity associated with clopidogrel or aspirin can also occur in TAVI patients4, although its clinical correlations remain unclear; c) the impact of old versus newer percutaneous valve technologies remains elusive –for example, inclusion of an additional skirt to reduce the frequency of paravalvular leak may potentially increase thrombogenicity.
Forthcoming studies
Further evidence is therefore needed to conclude firmly that DAPT is futile compared with aspirin alone: ongoing trials will help to clarify this debated issue. The Aspirin Versus Aspirin+ClopidogRel Following Transcatheter Aortic Valve Implantation (ARTE) pilot trial (NCT01559298) assesses the efficacy of aspirin alone (80 mg/day for at least six months) versus aspirin (80 mg/day for at least six months)+clopidogrel (75 mg/day for three months) in preventing death, myocardial infarction, ischaemic stroke/transient ischaemic attack (TIA) or life-threatening/major bleeding (primary endpoint) at one year in 200 patients with no indication to OAC. The Dual Antiplatelet Therapy Versus Oral Anticoagulation for a Short Time to Prevent Cerebral Embolism After TAVI (AUREA) trial (NCT01642134) assesses the efficacy of DuoPlavin (aspirin 80 mg/day+clopidogrel 75 mg/day, for three months) compared with acenocumarol in preventing cerebral thromboembolism identified using magnetic resonance (primary endpoint) at three months in 124 patients with no indication to OAC. The Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation (POPular-TAVI) trial (NCT02247128) hypothesises that omitting clopidogrel in the first three months after TAVI has similar efficacy and greater safety compared with adding clopidogrel to aspirin or OAC. This is a multicentre open-label randomised all-comers trial comparing safety, net clinical benefit and efficacy of clopidogrel omission compared to aspirin (100 mg/day for at least one year)+clopidogrel (75 mg/day for three months) (cohort A) or OAC+clopidogrel (cohort B) in 1,000 patients over one year of follow-up. Future studies will also need to address multiple outstanding issues including the role of clopidogrel monotherapy, the need for a loading dose and the potential role of newer P2Y12 antagonists, prasugrel or ticagrelor.
The role of anticoagulation
Whether thrombi produced during and after TAVI have a platelet- or thrombin-based origin remains uncertain. Hence, antiplatelet-based strategies alone may still be suboptimal. Moreover, the need for OAC is also supported by the high burden of pre-existing and new-onset AF in TAVI patients, particularly since the large majority of these patients have a high CHA2DS2-VASc score. OAC in this context has great relevance in thrombosis prevention because new-onset and recurrent paroxysmal AF may be silent, clinically unrecognised and high risk unless specifically investigated2. Transcatheter valve thrombosis is rare but dangerous and may result in elevated transvalvular gradients requiring OAC. A recent study reviewed a total of 18 published cases (SAPIEN=17, CoreValve=1) and reported four new cases (SAPIEN=1, CoreValve=3)4, while a larger multicentre retrospective study analysed 4,266 patients, reporting 26 cases of transcatheter valve thrombosis (mean follow-up six months; SAPIEN=20, CoreValve=6)5. Clinical presentation was principally with dyspnoea and increased gradients, and anticoagulation therapy was effective in reducing gradients in the majority of patients within two months of treatment. The frequency of transcatheter valve thrombosis may be underestimated, however, since clinical signs and symptoms can be masked by comorbidities, and early follow-up echocardiography is not uniformly performed. Nonetheless, pannus formation or thrombosis should be suspected in patients with sudden elevation in valve gradient, prompting further investigation and therapy with OAC plus single antiplatelet therapy (SAPT) or DAPT. In case of failure, valve-in-valve TAVI or SAVR could be considered.
The concept that OAC could be important in TAVI patients was also discussed at EuroPCR 2015. Neumann presented the results of systematic computed tomography (CT) five days after TAVI (SAPIEN 3 prosthesis), demonstrating clinically silent valve leaflet thickening in 16/156 patients. Full OAC (INR 2.5-3.5) resulted in regression of these findings in 11 patients who underwent follow-up CT (mean 77 days). In the same session, Sondergaard presented data concerning valve leaflet motion assessed with CT approximately three months after TAVI (n=47) or SAVR (n=15), demonstrating that the frequency of reduced leaflet motion was similar with different types of valve (and procedure), and that the phenomenon was not associated with clinical events. Whilst these preliminary underpowered data findings await confirmation and more rigorous evaluation, they may open new perspectives on the post-procedural management of TAVI patients, underlining the need to consider a more liberal approach to the use of OAC after TAVI, even in the absence of known indications (AF, mechanic valves), and the utility of CT imaging during follow-up. Conversely, the necessity of this approach remains the subject of discussion since all patients were asymptomatic and no clinical events were reported or prevented.
Bleeding risks
A crucial aspect to be considered in the management of TAVI patients is the risk of bleeding –a frequent periprocedural complication after TAVI associated with worse prognosis1. However, major late bleeding events (>30 days) also significantly increase mortality in this population and have great clinical relevance6.
The low incidence of transcatheter valve thrombosis and high bleeding risk in most TAVI patients may not justify the routine use of OAC, but recent evidence supports the importance of OAC in some patients and future trials are needed. Accordingly, two multicentre randomised trials have recently been designed (Figure 1). GALILEO (Global multicenter, open-label, randomized, event-driven, active-controlled study comparing a rivAroxaban-based antithrombotic strategy to an antipLatelet-based strategy after transcatheter aortIc vaLve rEplacement (TAVR) to Optimize clinical outcomes) will compare rivaroxaban-based (rivaroxaban 10 mg/day long-term with aspirin 75-100 mg/day for three months) and antiplatelet-based strategies (aspirin 75-100 mg/day long-term with clopidogrel 75 mg/day for three months) after TAVI in patients without prior indication for anticoagulation. ATLANTIS (Anti-Thrombotic strategy to Lower All cardiovascular and Neurologic ischemic and hemorrhagic events after Trans-aortic valve Implantation for aortic Stenosis) will compare apixaban (5 mg bd or 2.5 mg bd in specific settings) with the standard of care, irrespective of need for oral anticoagulation.
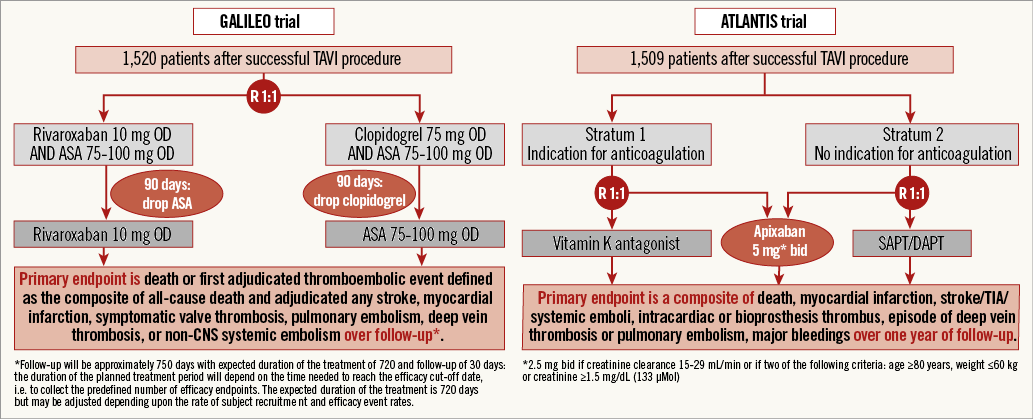
Figure 1. Flow diagram of the GALILEO and ATLANTIS trials.
Finally, there is a paucity of data and ongoing controversy concerning the appropriate antithrombotic regimen (and its duration) in AF patients undergoing TAVI. There are only individual reports concerning the use of triple therapy (DAPT+OAC) and no evidence regarding warfarin with one antiplatelet agent or warfarin alone. Indeed, American and Canadian guidelines discourage the use of triple therapy (Table 1). In AF patients undergoing stenting, the combination of OAC with one antiplatelet agent was associated with better safety outcomes (and no excess of ischaemic events) than triple therapy. However, the recent European consensus on AF treatment stated that VKA alone is the preferred option in patients undergoing TAVI with AF but no CAD, since the need for additional antiplatelet therapy remains uncertain. Conversely, patients who have undergone recent PCI should be treated similarly to those receiving stents outwith the context of TAVI, since specific robust data for TAVI are lacking and future trials needed.
In conclusion, the justification for currently recommended regimes of DAPT after TAVI has recently been questioned, while arguments supporting the potential benefits of OAC therapy have now emerged. Well designed and appropriately powered trials are strongly warranted.
Conflict of interest statement
The authors have no conflicts of interest to declare.

