Abstract
Percutaneous transcatheter structural heart interventions have considerably expanded within the last two decades, improving clinical outcomes and quality of life versus guideline-directed medical therapy for patients frequently ineligible for surgical treatment. Transcatheter structural heart interventions comprise valve implantation or repair and also occlusions of the patent foramen ovale, atrial septal defects and left atrial appendage. These procedures expose structural devices to arterial or venous blood flow with various rheological conditions leading to potential thrombotic complications and embolisation. Furthermore, these procedures may concern comorbid patients at high risk of both ischaemic and bleeding complications. This state-of-the-art review provides a description of the device-related thrombotic risk associated with these transcatheter structural heart interventions and of the current evidence-based guidelines regarding antithrombotic treatments. Gaps in evidence for each of the studied transcatheter interventions and the main ongoing trials are also summarised.
Various transcatheter interventions have been developed in the last three decades for the treatment of structural heart diseases. They all involve exposure of cardiac devices to blood flow and, therefore, thrombosis formation during the early healing phase, justifying preventive antithrombotic treatments. Various antithrombotic treatments are available such as single (SAPT) or dual antiplatelet therapy (DAPT) and oral anticoagulation (OAC), prescribed individually or in combination. However, the optimal antithrombotic regime per type of transcatheter structural heart intervention has not yet been developed. In this state-of-the-art review, we aim to summarise the current evidence and provide perspectives on the evolving field of antithrombotic therapy as used following transcatheter interventions.
Transcatheter aortic valve implantation
TAVI in patients without an indication for chronic oral anticoagulation
The optimal antithrombotic therapy post-transcatheter aortic valve implantation (TAVI) has been debated over the last decade12. Most TAVI patients are older and/or frail and thus prone to both thromboembolic and bleeding complications. Based on the protocols used in the first landmark randomised controlled trials (RCTs) comparing TAVI to surgery, transient DAPT was recommended by expert consensus3. This assumption was eventually discarded according to consistent findings from both observational studies and RCTs with increasing sample sizes and statistical power45678. In the cohort of the POPular TAVI trial, a total of 665 patients (mean age 81.0±5.9 years, 45.4% female, median Society of Thoracic Surgeons [STS] risk scores between 3.1 [interquartile range IQR 2.3-4.5] and 3.2 [IQR 2.2-4.8]) were randomised to receive either aspirin only or DAPT with aspirin and clopidogrel, with a loading dose the day prior to the procedure and continued for 3 months. Both primary endpoints, all bleeding and non-procedure-related bleeding over a 12-month period, were significantly reduced in the SAPT arm (risk ratio [RR] 0.57, 95% confidence interval [CI]: 0.42-0.77, and RR 0.61, 95% CI: 0.44-0.83, respectively). This gain in bleeding prevention was not offset by any significant increase in the rate of ischaemic complications, such as the non-hierarchical composite of cardiovascular (CV) death, ischaemic stroke, or myocardial infarction (MI), for which SAPT was demonstrated to be non-inferior to DAPT. Previous international guidelines by the European Society of Cardiology (ESC) and the American College of Cardiology/American Heart Association (ACC/AHA) proposed transient DAPT with class IIa and IIb recommendations, respectively910. Following the results of the POPular TAVI trial, the ESC updated its guidelines in 2021 to grant a class I, Level of Evidence A recommendation in favour of lifelong SAPT following TAVI in patients without any indication for chronic OAC1112 (Central illustration). Despite these findings, there are pending issues, as summarised in Figure 1.
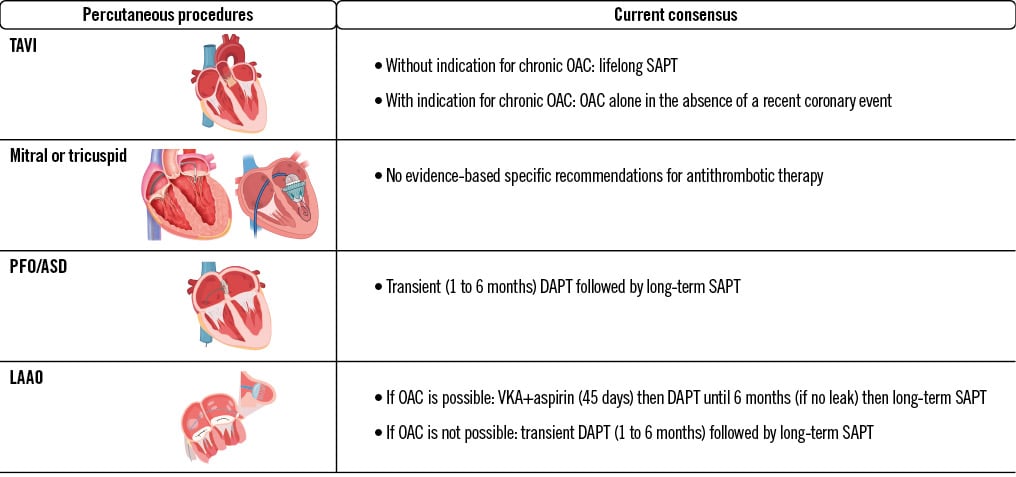
Central illustration. Guidelines and expert-based consensus on antithrombotic therapy for transcatheter structural heart intervention. ASD: atrial septal defect; DAPT: dual antiplatelet therapy; LAAO: left atrial appendage occlusion; OAC: oral anticoagulant; PFO: patent foramen ovale; SAPT: single antiplatelet therapy; TAVI: transcatheter aortic valve implantation; VKA: vitamin K antagonist
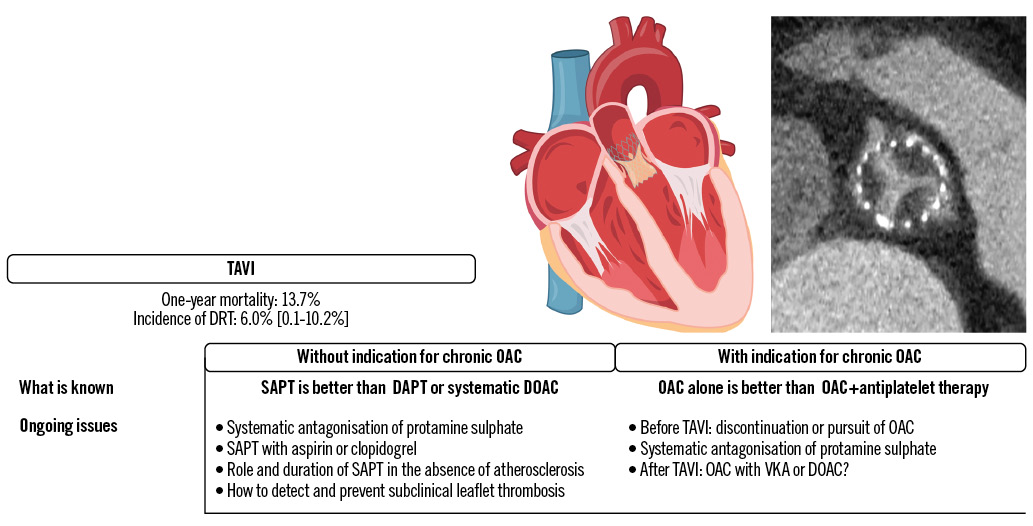
Figure 1. Main ongoing issues with TAVI antithrombotic strategies and related DRT. DAPT: dual antiplatelet therapy; DOAC: direct oral anticoagulant; DRT: device-related thrombosis; OAC: oral anticoagulant; SAPT: single antiplatelet therapy; TAVI: transcatheter aortic valve implantation; VKA: vitamin K antagonist
Subclinical leaflet thrombosis of percutaneous bioprosthetic aortic valves
Subclinical leaflet thrombosis (SLT) following TAVI has been the focus of intensive clinical research, especially with the increasing use of four-dimensional (4D) computed tomography (CT) scans, where it can manifest as hypoattenuated leaflet thickening (HALT) and/or reduced leaflet motion (RLM)13. In a recent meta-analysis, comprising 25 studies and 11,098 patients, the reported incidence of SLT was 6% overall and was significantly more frequent in case of an intra-annular valve (RR 2.03, 95% CI: 1.42-2.89) or treatment with SAPT or DAPT rather than OAC (RR 0.42, 95% CI: 0.29-0.61)14. SLT was associated with a significantly increased rate of the composite of stroke or transient ischaemic attack (TIA; RR 2.56, 95% CI: 1.60-4.09), consistent with previous meta-analyses, even when excluding periprocedural events14151617. The use of OAC following TAVI prevents or treats the occurrence of SLT, while the use of DAPT did not offer any improved protection compared to SAPT alone1819. The optimal type and duration of OAC to be initiated after a diagnosis of SLT remains unclear. It should be noted that current guidelines recommend anticoagulation in patients presenting with both HALT and RLM, leading to an elevated gradient, at least until resolution12. Both vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs) may be initiated, and repeat 4D-CT valvular imaging should be performed, usually after a 3- to 6-month period202122. Of note, SLT reoccurrence after OAC discontinuation has been reported, thus warranting prolonged monitoring23. This finding was part of the rationale behind the systematic use of a DOAC following TAVI in patients without any other indication for OAC, albeit unsuccessfully. The GALILEO trial, which compared a low dose of rivaroxaban (i.e., 10 mg q.d.) combined with 3 months of aspirin to 3-month DAPT followed by aspirin monotherapy, was prematurely interrupted because of the significant increase in all-cause and non-CV death associated with the former (hazard ratio [HR] 1.69, 95% CI: 1.13-2.53 and HR 2.67, 95% CI: 1.33-5.35, respectively)24. This mortality increase was likely driven by a higher risk of Valve Academic Research Consortium (VARC)-defined major bleeding (HR 2.02, 95% CI: 1.09-3.76); of note, there was no significant gain in terms of ischaemic events, including ischaemic stroke (HR 1.28, 95% CI: 0.73-2.23). The ATLANTIS trial evaluated the use of apixaban compared to standard of care, which consisted of SAPT/DAPT in patients not requiring chronic OAC (stratum 2) and of VKA in patients with an indication for chronic OAC (stratum 1)25. Although the study reported apixaban to be non-inferior to standard of care for the primary endpoint − a composite of ischaemic and bleeding events − in the whole population, in stratum 2, it was also associated with a significant increase in the risk of all-cause and non-CV death (HR 1.86, 95% CI: 1.04-3.34 and HR 2.99, 95% CI: 1.07-8.35, respectively), albeit without a significant increase in the risk of bleeding in contrast to what was observed in the GALILEO trial. In dedicated imaging substudies of both the GALILEO and the ATLANTIS trials, the use of DOACs significantly reduced the risk of SLT, compared to an antiplatelet-based regimen, in patients without an indication for chronic OAC but failed to reduce the risk of clinical stroke (Figure 2)2627. More recently, the ADAPT-TAVR trial randomised 229 patients to be treated with either edoxaban or 6-month DAPT following TAVI28. The primary endpoint, which was the incidence of SLT on a 4D-CT scan at 6 months, was only numerically reduced in the edoxaban group (9.8% vs 18.4%; p=0.076). Consequently, the routine use of DOACs following TAVI in patients without an indication for OAC has a class III, Level of Evidence B recommendation in the latest European guidelines12. Of note, the risk of very late (i.e., >1 year after the procedure) clinically significant valve thrombosis according to the VARC-3 criteria affected 2.1% of the low-risk patients treated with TAVI in the PARTNER 3 Trial, a significantly increased risk compared to surgical aortic valve replacement (HR 8.72, 95% CI: 1.12-68.12)29. As TAVI indications are progressively expanded to younger and lower-risk populations, future studies assessing systematic DOACs, perhaps with lower dosage, compared to antiplatelet therapy could be of interest for the prevention of SLT. Furthermore, ongoing phase III RCTs are evaluating factor XIa inhibitors in patients with various indications, such as atrial fibrillation (AF) with embolic complication or in the setting of coronary artery disease (CAD)30. Future studies evaluating the use of factor XIa inhibitors to prevent SLT after TAVI would be of interest, but the results of studies for other indications must be awaited first.
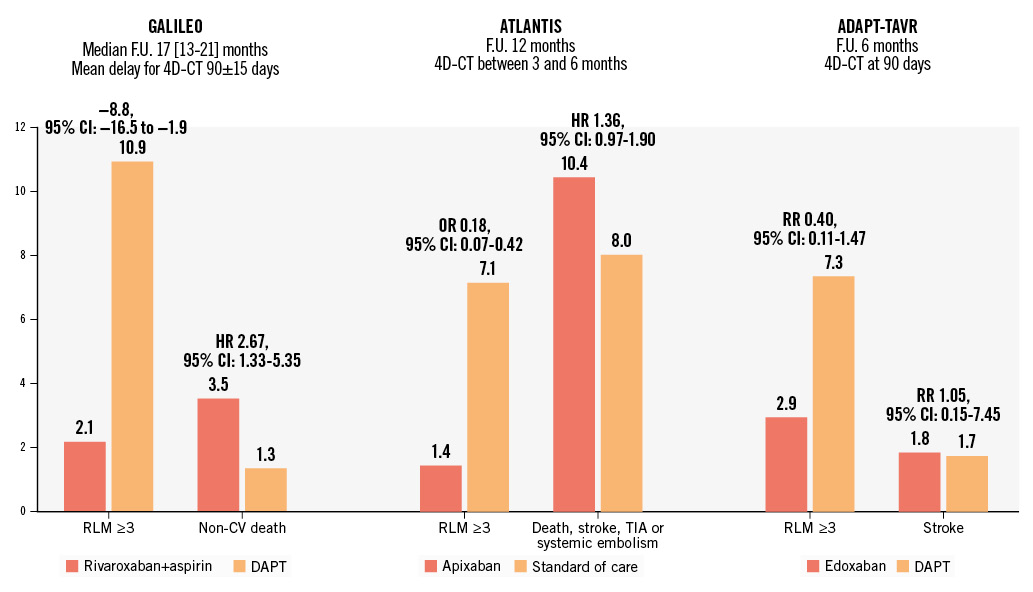
Figure 2. Dichotomy of the effect of direct oral anticoagulation after TAVI on imaging and clinical outcomes. 4D-CT: four-dimensional computed tomography; CI: confidence interval; CV: cardiovascular; DAPT: dual antiplatelet therapy; F.U.: follow-up; HR: hazard ratio; OR: odds ratio; RLM: reduced leaflet motion; RR: risk ratio; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack
Which antiplatelet to keep?
Lifelong SAPT following TAVI is recommended in patients without an indication for chronic OAC. SAPT is overwhelmingly represented by low-dose aspirin, as it is for percutaneous coronary intervention. So far, in the setting of TAVI, only 1 observational study from the Japanese OCEAN-TAVI registry has compared clopidogrel to aspirin in patients with and without an indication for chronic OAC31. After propensity matching, the median age was 85 years, the majority of patients were female, and the mean STS score was 5.8% in patients without anticoagulation and 7.5% in patients with anticoagulation. In the former group, the use of clopidogrel was associated with a reduction of CV death at 2 years (HR 0.81, 95% CI: 0.25-2.65), albeit without any significant difference in the risk of stroke or life-threatening or major bleeding, which most frequently occurred within 30 days of the procedure. Other observational studies comparing SAPT with clopidogrel versus aspirin following TAVI in other non-Asian populations are certainly warranted to confirm these results.
The REAC-TAVI trial demonstrated that DAPT with aspirin and ticagrelor led to improved antiplatelet activity compared to aspirin and clopidogrel, which was frequently associated with residual high platelet reactivity32.
No antithrombotic treatment
Another observational study from the OCEAN-TAVI registry evaluated patients discharged without any antithrombotic therapy following TAVI33. Out of 3,575 patients discharged without OAC or procedural complication, 293 (8.2%) were discharged without antiplatelet therapy per the attending physician’s decision. As it could have been expected, the main motivation behind this antithrombotic abstinence was the perceived risk of bleeding of these patients, who were nonetheless younger and with less frequent chronic kidney disease or anaemia than patients discharged with antiplatelet therapy. After 3 years of follow-up, the adjusted risk of all bleeding was significantly reduced in the absence of any antiplatelet therapy compared with DAPT (HR 0.51, 95% CI: 0.27-0.95) and numerically reduced compared to SAPT (HR 0.63, 95% CI: 0.33-1.19), without any significant difference in terms of mortality or ischaemic complications. Based on these results, the Non-antithrombotic Therapy After Transcatheter Aortic Valve Implantation Trial (ClinicalTrials.gov: NCT06007222) was initiated in 2023 to compare aspirin-only to no antithrombotic therapy in 360 patients undergoing transfemoral TAVI. The primary endpoint is a composite of death, MI, stroke and bleeding (type 1 to 4) according to the VARC-3 criteria between 1 and 3 years of follow-up. Further studies confirming these results in other populations are warranted34.
What is next?
Two ongoing trials are comparing the impact of SAPT with aspirin versus antiplatelet P2Y12 inhibitors on imaging and clinical outcomes. The ACLO-TAVR Trial was initiated in 2023 in Korea (ClinicalTrials.gov: NCT05493657) and will include 230 patients initially treated with 1-month DAPT followed by either aspirin or clopidogrel only, and the primary endpoint is the incidence of SLT on cardiac CT at 3 months. The REAC-TAVI2 trial (ClinicalTrials.gov: NCT05283356) is randomising 1,206 patients with diabetes mellitus and prior documented coronary or peripheral artery disease to be treated, after a successful TAVI, with SAPT with either low-dose aspirin alone or ticagrelor 60 mg b.i.d. alone. The primary endpoint is a composite of death, stroke, transient ischaemic attack, MI, progressive angina leading to emergency evaluation, rehospitalisation or new coronary angiography, valve thrombosis, claudication, acute limb ischaemia leading to hospitalisation, or any bleeding. On the other hand, a third, recently initiated, trial is evaluating the interest of an antithrombotic strategy based on the results of systematic 4D-CT scan evaluation and the clinical characteristics of the patients. The POP ATLANTIS (ClinicalTrials.gov: NCT06168370) international, multicentre, event-driven, open-label trial will include 2,496 patients after a successful TAVI procedure. Patients are henceforth treated with aspirin for 3 months, after which they are randomised to either standard of care or an antithrombotic strategy, based on the results of a 4D-CT scan. In the absence of subclinical leaflet thrombosis, patients will be treated with SAPT or no antithrombotic therapy, in the presence or absence of vascular disease, respectively. In case of SLT, patients will be switched to apixaban with another 4D-CT scan evaluation after 6 months, at which point apixaban will be interrupted or continued according to the regression or persistence of the bioprosthesis thrombosis, respectively. The co-primary endpoints will be the composite of thromboembolic and all bleeding events.
TAVI in patients with an indication for chronic oral anticoagulation
Patients requiring chronic treatment with OAC currently represent approximately 40% and 16% of high-risk and low-risk patients undergoing TAVI, respectively135. The issue of concomitant prescription of antiplatelet therapy in this population was addressed by cohort B of the POPular-TAVI trial, which reported the addition of clopidogrel to OAC to be associated with a significantly increased risk of all bleeding (RR 0.63, 95% CI: 0.43-0.90) and non-procedure-related bleeding (RR 0.64, 95% CI: 0.44-0.92)36. Notwithstanding, some major issues remain.
To continue or to stop oral anticoagulation prior to TAVI?
Current expert-based guidelines advise the interruption of OAC in patients at high risk of bleeding undergoing interventions but do not address the specific issue of patients undergoing transfemoral TAVI37. Other expert-based consensus recommend the use of unfractionated heparin (UFH) sulphate aiming for an activated clotting time (ACT) >250-300 sec for periprocedural anticoagulation38. However, some observational data have reported the continuation of OAC to be safe and potentially associated with a reduction of the VARC-2 safety criteria39. The ongoing POPular PAUSE TAVI will be the first RCT to compare the continuation versus discontinuation of OAC, stratified on the use of VKA or DOAC and on the incidence of CV death, stroke, MI, major vascular complication and type 2-4 bleeding, as defined by the VARC-3 criteria, within 30 days of the TAVI procedure40.
Which oral anticoagulant to use after TAVI?
The use of a DOAC following TAVI, mostly to prevent AF-related thromboembolic complications, has increased 5.5-fold between 2013 and 2018 in the STS/ACC Transcatheter Valve Therapy (TVT) Registry41. The main observational studies comparing DOAC to VKA after TAVI are detailed in Table 1. Overall, the use of a DOAC seems to be safe, potentially reducing bleeding and ischaemic complications, albeit with some conflicting data. So far, only 2 RCTs have compared a DOAC to VKA. Stratum 1 of the ATLANTIS trial did not report significant differences between apixaban and VKA regarding either ischaemic or bleeding complications25. The ENVISAGE-TAVI AF trial randomised 1,426 patients following successful TAVI to treatment with edoxaban or VKA. Although edoxaban was non-inferior to VKA for the primary endpoint − a composite of death, MI, stroke, systemic thromboembolism, valve thrombosis, or major bleeding (HR 1.05, 95% CI: 0.85-1.31) − it was associated with a higher risk of major bleeding (HR 1.40, 95% CI: 1.03-1.91)42, mainly accounted for by the concomitant use of SAPT and DAPT in 57.8% and 12.6% of the population, respectively, and the frailty of the population, which met the criteria for a reduced dose of edoxaban in 46.4% of the cases. Notwithstanding these results, and while waiting for updated recommendations by international guidelines, the use of DOAC, with a dosage adapted to the patients’ frailty and without systematic use of concomitant antiplatelets, seems reasonable in patients following TAVI.
Table 1. Main observational studies comparing DOAC to VKA after TAVI.
| Registry or first author and year of publication | Years of recruitment | Location | Number of patients included | Means of comparison | Main results concerning the use of DOAC versus VKA |
|---|---|---|---|---|---|
| SwissTAVI, 202490 | 2007-2021 | Switzerland | Overall: 3,867VKA: 1,536DOAC: 2,304 | Propensity score matching | Increased risk of 5-year mortality (HR 1.25, 95% CI: 1.11-1.40) and lower risk of non-procedure-related disabling stroke (HR 0.64, 95% CI: 0.46-0.90) with VKA |
| GARY, 202391 | 2011-2019 | Germany | Overall: 16,974VKA: 11,333 (66.8%)DOAC: 5,641 (33.2%) | Propensity score matching | No significant differences in the risk of 1-year death (HR 0.95, 95% CI: 0.88-1.01) or cardiac and cerebrovascular event (HR 0.93, 95% CI: 0.86-1.01) |
| STS/ACC TVT, 202241 | 2013-2018 | USA | Overall: 21,131VKA: 13,004 (61.5%)DOAC: 8,127 (38.5%) | Adjusted Cox proportional hazard model | Reduced risk of 1-year all bleeding (HR 0.81, 95% CI: 0.75-0.89), intracranial haemorrhage (HR 0.54, 95% CI: 0.33-0.87) and death (HR 0.92, 95% CI: 0.85-1.00) with DOAC |
| Butt et al, 202192 | 2012-2017 | Denmark | Overall: 735VKA: 516 (70.2%)DOAC: 219 (29.8%) | Adjusted Cox proportional hazard model | No significant differences in the 3-year risk of arterial thromboembolism (HR 1.23, 95% CI: 0.58-2.59), bleeding (HR 1.14, 95% CI: 0.63-2.06) or death (HR 0.93, 95% CI: 0.61-1.40) |
| FRANCE-TAVI and FRANCE 2, 202193 | 2010-2017 | France | Overall: 24,581VKA: 15,619 (63.5%)DOAC: 8,962 (36.5%) | Propensity score matching | Reduced risk of 3-year death (HR 0.73, 95% CI: 0.60-0.89) and major bleeding including intracranial haemorrhage (HR 0.61, 95% CI: 0.44-0.85) with DOAC |
| OCEAN-TAVI, 202094 | 2013-2017 | Japan | Overall: 403VKA: 176 (43.7%)DOAC: 227 (56.3%) | Propensity score matching (IPTW) and adjusted Cox proportional hazard model | After a median follow-up of 568 days, significant reduction in the risk of death (IPTW-adjusted HR 0.53, 95% CI: 0.29-0.96 and Cox-adjusted HR 0.39, 95% CI: 0.20-0.75) with DOAC |
| Jochheim et al, 201995 | 2007-2017 | 4 centres in Germany and Italy | Overall: 962VKA: 636 (66.1%)DOAC: 326 (33.9%) | Propensity score matching (IPTW) | Increase in the risk of 1-year death, MI or cerebrovascular event (adjusted HR 1.44, 95% CI: 1.00-2.07) with DOAC |
| CI: confidence interval; DOAC: direct oral anticoagulant; HR: hazard ratio; IPTW: inverse probability of treatment weighting; MI: myocardial infarction; STS/ACC TVT: Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy; TAVI: transcatheter aortic valve implantation; VKA: vitamin K antagonist | |||||
Place of concomitant percutaneous left atrial appendage occlusion
The WATCH-TAVR trial recently compared TAVI with concomitant percutaneous left atrial appendage occlusion (LAAO) to TAVI+medical therapy in 349 patients (mean age 81.1±7.2 years, mean CHA2DS2-VASc and HAS-BLED scores 4.9±1.2 and 3.0±1.1, respectively) with AF43. The combined procedures were non-inferior to TAVI+medical therapy for the composite primary endpoint of mortality, stroke and major bleeding at 2 years (HR 0.86, 95% CI: 0.60-1.22; p-value for non-inferiority<0.001) but were not superior (p-value for superiority=0.40) and were associated with an increased risk of arterial or venous thrombosis or embolism (HR 5.03, 95% CI: 1.47-17.26), mainly driven by venous thrombosis.
PERIPROCEDURAL CONSIDERATIONS FOR TAVI
Expert-based guidelines acknowledge the possibility of heparin antagonisation using protamine sulphate in case of a transapical approach and in case of major bleeding or vascular complications following a transfemoral approach38. One large retrospective study including 873 patients from 2013 to 2018 reported systematic use of protamine sulphate to be associated with a significant reduction in the risk of death, life-threatening or major bleeding complications (odds ratio [OR] 0.24, 95% CI: 0.10-0.58), as well as a shorter length of hospital stay, without any significant ischaemic offset44. These results were confirmed by a recent multicentre observational registry where full heparin antagonisation resulted in significantly lower rates of life-threatening and major bleeding after TAVI compared with partial heparin reversal45. However, in a single-centre RCT with a limited sample size of 100 patients, systematic antagonisation of protamine sulphate failed to reduce the rate of major and life-threatening bleeding within 48 hours of the procedure (OR 0.48, 95% CI: 0.2-1.2)46. The POPular ACE TAVI (ClinicalTrials.gov: NCT05774691) was recently initiated and will include 1,000 patients undergoing transfemoral TAVI to be treated with routine or selective (i.e., in case of life-threatening bleeding) protamine administration (ratio of 1 mg per 100 IU of UFH). The primary outcomes are the composite of cardiovascular death or type 1 to 4 bleeding, according to the VARC-3 criteria. Consistently, the ATLANTIS PROTAMINE trial (ClinicalTrials.gov: NCT06215378) will randomise 940 patients undergoing transfemoral TAVI in France to be treated with systematic protamine administration versus selected protamine administration limited to cases of significant bleeding complication. The primary endpoint will include the composite of VARC-3 defined all-cause mortality, type 2, 3 or 4 bleeding, major or minor vascular complications, stroke or TIA, myocardial infarction or any red blood transfusion.
Antithrombotic therapy after percutaneous mitral and tricuspid interventions
Transcatheter mitral valve implantation (TMVI) is considered a high-risk situation for device-related thrombosis (DRT) due to the transmitral low-flow state and patient-related risk factors such as advanced age, concomitant AF, altered ventricular function or hypercoagulable state47. A relatively large single-centre registry of 130 patients undergoing TMVI with the SAPIEN device (Edwards Lifesciences) reported a 1-year cumulative rate of transcatheter heart valve thrombosis of 11.1% (95% CI: 6.5-18.2%), occurring mostly during the index hospitalisation or within the first 3 months post-procedure, despite an antithrombotic regimen of VKA, with a target international normalised ratio (INR) of 2-3, and low-dose aspirin48. Risk factors for device thrombosis were the presence of in-hospital haemodynamic shock due to acute thrombosis, male sex and the lack of early preventive OAC (i.e., within 3 months). Another observational registry evaluating the Tendyne prosthesis (Abbott) reported an incidence of device thrombosis of 6% at 1 year. It should be noted that the postoperative antithrombotic protocol was modified during the study course, with a switch from low-dose aspirin to the systematic use of VKA with a target INR of 2.5 to 3.5 for ≥3 months. Valve thrombosis may also be a concern in case of valve-in-valve TMVI. A recent, relatively large single-centre registry reported DRT incidence at 2.5%, 5.9% and 8.4% at 1 month, 1 year and beyond 1 year, respectively, supporting the use of OAC after valve-in-valve TMVI49.
Mitral transcatheter edge-to-edge repair (TEER) is a more common intervention, compared to TVMI. In the landmark COAPT Trial, comparing TEER with the MitraClip device (Abbott) to guideline-directed medical therapy for the treatment of symptomatic severe secondary mitral regurgitation, the protocol mandated discontinuation of chronic OAC ≥3 days prior to the procedure, periprocedural UFH with a target ACT >250 seconds and a loading dose of clopidogrel before or immediately after the procedure50. OAC was resumed when needed, or low-dose aspirin was to be initiated for 6 months. Although the trial did not adjudicate device thrombosis as safety endpoints, there were 2 (0.7%) centre-reported cases of “thrombosis in device” during the 5-year follow-up in the experimental group (Figure 3). Consistently, only a limited number of case reports about TEER DRT have been reported so far, mostly occurring during the index procedure rather than afterwards51.
Tricuspid TEER was recently demonstrated to be safe and effective versus optimal medical therapy52. The protocol mandated resuming OAC after the procedure when indicated, i.e., in at least 90% of the overall population, or the use of aspirin with or without clopidogrel for at least 6 months. No case of DRT was adjudicated by the central core lab of the study within 30 days of the procedure. Consistently, the large real-world bRIGHT post-approval study of 511 patients undergoing TEER with the TriClip (Abbott) did not report any DRT53.
Large RCTs evaluating transcatheter tricuspid valve implantation (TTVI) are ongoing. The large observational VIVID registry reported outcomes following TTVI, with either the Melody (Medtronic) or SAPIEN valve, in patients with a prior tricuspid prosthesis or ring54. Of the 306 patients included, a total of 8 presented with device thrombosis over the median 15.9 months of follow-up, of which 3 occurred within several days of the procedure, 2 within months and 3 after 6 months of the procedure. Most of the cases occurred in patients not treated with OAC at the time of diagnosis. The recent, prospective, international TRISCEND Study reported outcomes following TTVI with the EVOQUE (Edwards Lifesciences) device in patients with ≥moderate symptomatic tricuspid regurgitation55. There was no reported case of device-related pulmonary embolism, although the specific rate of DRT was not detailed in a population of patients overwhelmingly treated with OAC.
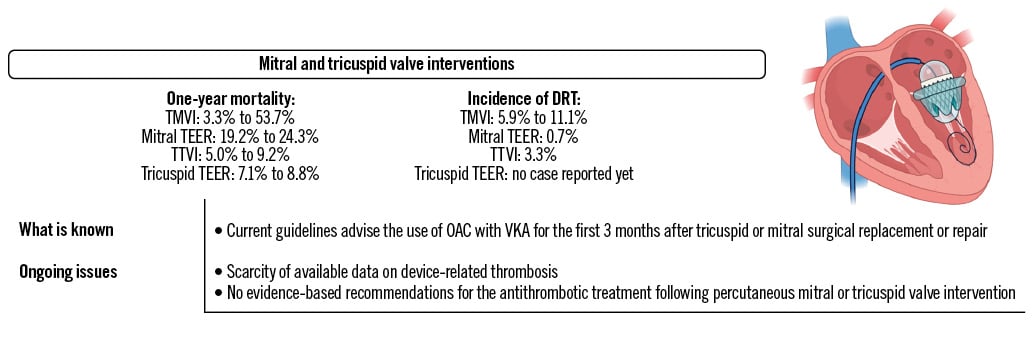
Figure 3. Main ongoing issues with mitral and tricuspid valve interventions’ antithrombotic strategies and related DRT. DRT: device-related thrombosis; OAC: oral anticoagulant; TEER: transcatheter edge-to-edge repair; TMVI: transcatheter mitral valve implantation; TTVI: transcatheter tricuspid valve implantation; VKA: vitamin K antagonist
Scarcity of data on device-related thrombosis and postprocedural antithrombotic treatment
Data on DRT are scarce, leading to significant differences in real-world practices after these interventions, which are aimed at different patient populations, with significant variations in term of rheological conditions and thrombotic risk56. The rate of DRT seems to be significantly higher in case of percutaneous mitral or tricuspid replacement rather than repair, although prothrombotic comorbidities, such as older age, AF, and altered ventricular function, are quite common in both situations. Current international guidelines recommend the use of VKA for the first 3 months after tricuspid or mitral surgical repair or bioprothesis implantation and continuation of DOACs rather than VKAs when needed. The indication for long-term OAC may be significantly more frequent for these procedures compared to TAVI, with more than half of the COAPT population and 90% of the TRILUMINATE patients presenting with a history of AF5052. Of note, a post hoc analysis of the COAPT Trial reported that the baseline use of OAC was associated with a reduction in the risk of cerebrovascular events in patients treated with mitral TEER, conversely to what was observed among patients treated with guideline-directed medical therapy alone57. Whether a part of this beneficial effect could be linked to a reduction of mitral TEER-related DRT remains to be investigated. Only limited data comparing VKAs to DOACs in such patients are available. A recent single-centre retrospective observational study which included 156 patients undergoing TMVI between 2011 and 2023 reported the use of DOACs to be associated with a significant reduction of the length of hospital stay, as well as the risk of any bleeding at 1 year in multivariate analysis (adjusted HR 0.21, 95% CI: 0.06-0.74), without significant differences regarding thrombotic (i.e., valve thrombosis or stroke) events (adjusted HR 1.23, 95% CI: 0.42-3.65)58. Following mitral TEER, DOACs seem to be more frequently prescribed than VKAs, based on large national claims databases5659. One retrospective study compared DOACs to VKAs after surgical or percutaneous mitral valve repair among 1,178 patients with AF and reported, after propensity score matching, a lower risk of mortality (HR 0.67, 95% CI: 0.55-0.82), ischaemic stroke (HR 0.72, 95% CI: 0.52-1.00) and bleeding (HR 0.79, 95% CI: 0.63-0.99) with DOACs60. Larger observational studies and dedicated RCTs determining the optimal antithrombotic therapy to be prescribed following transcatheter mitral or tricuspid valve repair or replacement that include patients at high risk of bleeding are certainly warranted. Finally, the role of concomitant percutaneous LAAO closure among high bleeding risk patients who cannot be treated with long-term OAC remains to be investigated.
Patent foramen ovale or atrial septal defect percutaneous closure
There is a dearth of data from RCTs evaluating periprocedural and postprocedural antithrombotic therapy after patent foramen ovale (PFO) or atrial septal defect (ASD) closure. Expert consensus advocates transient (i.e., 1 to 6 months) DAPT followed by long-term SAPT6162, as used in RCTs, with the aim of preventing device thrombosis during endothelialisation, a process that may be completed within 3 to 6 months after implantation and is potentially impacted by various factors including nickel hypersensitivity6364. A single-centre observational study performed 2 decades ago reported an incidence of 2.5% during the first 6 months, occurring mostly within the first 4 weeks, using systematic transoesophageal echocardiography65. However, most of the devices used in this study are no longer available. Interestingly, there was only 1 case (0.2% of the experimental group) of device-related cardiac thrombus reported in the RESPECT trial, 2 cases (0.5% of the experimental group) in the REDUCE trial, and none in the CLOSE or DEFENSE PFO trials66. The CANOA randomised double-blind trial compared 3-month DAPT with clopidogrel to aspirin and placebo following ASD closure in 171 patients and reported a significant impact of clopidogrel on the primary endpoint of the number of migraine days per month within 3 months of the procedure, without any significant impact in terms of ischaemic and bleeding events67.
How many antiplatelet agents, if any, should be prescribed, and for how long after PFO or ASD closure?
In an observational study, DAPT for 3 months versus SAPT after PFO closure among 1,532 patients (median age: 49 [IQR 40-57] years, 42.2% female) had no significant benefit on the composite of death, stroke, TIA, peripheral arterial embolism, MI, or Bleeding Academic Research Consortium (BARC) type 2 or more bleeding during 5 years of follow-up (HR 0.96, 95% CI: 0.55-1.69)66. Although these results warrant further confirmation, they challenge the current recommendations (Figure 4). In fact, stroke recurrence following PFO closure seems to be more related to other risk factors, such as persistence of significant right-to-left intracardiac shunt and comorbidities such as diabetes mellitus, age, or sex, rather than the use of DAPT compared to SAPT686970. Consistently, although current recommendations advise for the continuation of long-term SAPT after DAPT interruption, some observational studies have reported the possibility of interrupting all antithrombotic treatment after 6 months without any alarming signals7172. As evidence-based recommendations are lacking, clinical practices may significantly vary according to the medical speciality of the attending physician, with interventional cardiologists more likely to recommend no long-term antithrombotic treatment after PFO closure, compared to stroke neurologists, as reported by a national survey performed in the USA73. The ongoing HALTI Trial (ClinicalTrials.gov: NCT04475510) is a single-arm observational study including patients aged ≤60 years, with 1 or fewer cardiovascular risk factors and without thrombophilia, moderate-to-severe residual right-to-left shunt or AF. In this study, patients discontinue all antiplatelet treatment 1 year after the procedure and undergo clinical and cerebral imaging evaluation at 12 and 24 months to search for any new ischaemic lesions.
Figure 4. Main ongoing issues with PFO/ASD antithrombotic strategies and related DRT. ASD: atrial septal defect; DAPT: dual antiplatelet therapy; DRT: device-related thrombosis; PFO: patent foramen ovale; SAPT: single antiplatelet therapy
Antithrombotic therapy after left atrial appendage occlusion
Left atrial appendage occlusion was initially developed as an alternative to VKAs in patients with a relatively low risk of bleeding74. For WATCHMAN devices (Boston Scientific), the postprocedural antithrombotic regimen approved by the U.S. Food and Drug Administration (FDA) consists of VKA/DOAC with aspirin for 6 weeks or until adequate left atrial appendage (LAA) sealing (i.e., without ≥5 mm leak), followed by DAPT with clopidogrel up to 6 months, and then lifelong aspirin monotherapy. The National Cardiovascular Data Registry suggests a lack of adherence to such protocol75. In Europe, LAAO is considered for patients with AF who have contraindications for long-term OAC, with a class IIb recommendation, and may be considered, based on experts’ consensus, in patients with a high risk of bleeding or willing to be treated with OAC7476. DAPT for between 1 and 6 months, according to the bleeding risk, followed by long-term aspirin or clopidogrel may be considered74. DRT is a complication affecting <5% of patients undergoing LAAO but is associated with a 4- to 5-fold increase in the risk of ischaemic events (Figure 5)76777879. Most of the cases of DRT seem to occur within 90 days of the procedure4950. However, in the large international LAAC-DRT and EUROC-DRT registries, late (i.e., >6 months) cases of DRT represented 17.9% and 36.3% of the total, respectively808182. The two main LAAO devices commercially available, the WATCHMAN and Amulet (Abbott) occluder devices, were compared in the SWISS-APERO trial and the larger AMULET IDE Trial8384. Overall, the rates of DRT varied according to the timing and the methods of assessment. In the SWISS-APERO trial, the recommended antithrombotic therapy was aspirin combined with clopidogrel or OAC for 3 months followed by aspirin alone for the remaining 9 months, but this was ultimately left to the discretion of the local physician. A cardiac computed tomography angiography was performed at 45 days and 13 months and centrally adjudicated; a rate of definite or possible DRT of 3.7% was reported for Amulet devices and 9.9% for WATCHMAN devices (RR 0.38, 95% CI: 0.12-1.17), and 2.4% for Amulet devices and 3.8% for WATCHMAN devices (RR 0.63, 95% CI: 0.11-3.70), respectively8485. At 45 days, a transoesophageal echocardiography was also performed, and a rate of DRT of 2.1% was reported for Amulet devices and 5.5% for WATCHMAN devices (RR 0.38, 95% CI: 0.08-1.93). In the AMULET IDE Trial, the FDA-approved regimen was recommended for patients treated with the WATCHMAN devices, while patients treated with the Amulet devices were recommended to be treated with aspirin plus clopidogrel or OAC at the discretion of the investigator. The rate of DRT at 18 months was 3.3% for the Amulet devices and 4.5% with the WATCHMAN devices83.
Figure 5. Main ongoing issues with LAAO antithrombotic strategies and related DRT. DAPT: dual antiplatelet therapy; DOAC: direct oral anticoagulant; DRT: device-related thrombosis; FDA: Food and Drug Administration; LAAO: left atrial appendage occlusion; OAC: oral anticoagulant; SAPT: single antiplatelet therapy; VKA: vitamin K antagonist
Which antithrombotic regimen should be used after LAAO when bleeding risk is high?
Post-LAAO antithrombotic treatment directly affects the risk of DRT and subsequent ischaemic complications. However, data mostly derived from observational studies and a recent network meta-analysis of 41 studies comprising 12,451 patients reported monotherapy with DOACs to have the highest likelihood of reducing the incidence of thromboembolic events and major bleeding, while DAPT was associated with a lower incidence of thromboembolic events compared to SAPT86. In a prospective non-randomised study, a half-dose of DOACs was associated with a significant reduction in the risk of DRT and thromboembolic and bleeding events compared to the FDA-approved regimen (HR 0.10, 95% CI: 0.02-0.43)87. The exploratory RCT ADRIFT compared 3-month DAPT and rivaroxaban alone (either 10 mg q.d. or 15 mg q.d.)88. The study was not powered to evaluate a clinical endpoint, but the only two cases of DRT confirmed by the blinded imaging core laboratory occurred in the DAPT group. Finally, the ADALA trial compared low-dose apixaban (i.e., 2.5 mg b.i.d.) to 3-month DAPT89. The study was prematurely interrupted after the inclusion of only 90 of the planned 160 patients and reported low-dose apixaban to be associated with a significant reduction in the composite of thromboembolic events, device thrombosis and major bleeding at 3 months (HR 0.19, 95% CI: 0.04-0.88). Table 2 details the main ongoing RCTs evaluating post-LAAO antithrombotic regimens.
Table 2. Main ongoing randomised controlled trials evaluating antithrombotic regimens after LAAO.
| Study title, clinical trial registration | Number of planned inclusions | Masking | Antithrombotic regimen evaluated | Primary endpoint |
|---|---|---|---|---|
| ANDESNCT03568890 | 510 | Open-label | Randomisation is performed after successful LAAO to either:- normal dosage of DOAC according to guidelines for 8 weeks- DAPT for 8 weeks | DRT evaluated by TOE at 8 weeks after the procedure |
| SAFE-LAAC NCT03445949 | 200 | Open-label | Randomisation is performed after successful LAAO to either:- DAPT for 30 days followed by SAPT- DAPT for 6 months | Composite of all-cause or CV death, ischaemic stroke, TIA, peripheral embolism, MI, or left atrial appendage thrombus at 17 months after randomisation |
| ASPIRIN-LAAO NCT03821883 | 1,120 | Double-blinded | Randomisation is performed 6 months after successful LAAO to either:- aspirin- placebo | Number of participants with CV death, stroke, systemic embolism, acute coronary syndrome, coronary or periphery artery disease requiring revascularisation, and major bleeding at 24 months after randomisation |
| FADE-DRT NCT04502017 | 360 | Open-label | Randomisation is performed after successful LAAO to either:- U.S. FDA-approved regimen- OAC for 6 weeks, followed by DAPT (for clopidogrel responder) or aspirin+half-dose OAC (for clopidogrel non-responders) for 6 months, then aspirin alone- half-dose DOAC | - Composite of stroke, systemic embolism, and DRT at 1 year- Composite of major bleeding at 1 year |
| RECORD-III NCT05960721 | 4,220 | Open-label | Randomisation is performed after successful LAAO to either:- low dose of rivaroxaban based on HAS-BLED score. If score <3, 15 mg q.d. for 3 months, then 10 mg q.d.; if ≥3, 10 mg q.d. for 3 months, then 2.5 mg b.i.d.- guideline-determined medication therapy based on HAS-BLED score: if score <3, rivaroxaban 15 mg q.d.+aspirin for 3 months then aspirin alone; if ≥3, DAPT for 3 months then aspirin alone | Rate of the composite endpoint of death, stroke, systemic embolism, and BARC type 3 or 5 bleeding events at 24 months from randomisation |
| ARMYDA-AMULETNCT05554822 | 574 | Open-label | Randomisation is performed after successful LAAO to either:- aspirin alone- DAPT for 3 months then aspirin alone | Composite of death, DRT (at 3- or 6-month TOE), ischaemic stroke, systemic embolism, or BARC ≥3 bleeding at 6 months after the procedure |
| Efficacy of Different Anti-Thrombotic Strategies on the Incidence of Silent Cerebral Embolism After Percutaneous Left Atrial Appendage Occlusion: a Randomized Control TrialNCT05671276 | 150 | Open-label | Randomisation is performed 45 days after successful LAAO to either:- DAPT from 45 days to 6 months then aspirin alone- rivaroxaban 10 q.d. | Incidence of new silent cerebral embolism by cerebral magnetic resonance imaging at 6 months |
| BARC: Bleeding Academic Research Consortium; CV: cardiovascular; DAPT: dual antiplatelet therapy (aspirin+clopidogrel); DOAC: direct oral anticoagulant; DRT: device-related thrombosis; FDA: Food and Drug Administration; LAAO: left atrial appendage occlusion; MI: myocardial infarction; OAC: oral anticoagulant; SAPT: single antiplatelet therapy; TIA transient ischaemic attack; TOE: transoesophageal echocardiography | ||||
Conclusions
Much more remains to be done in the field of antithrombotic therapy for transcatheter interventions to better determine the incidence and risk factors of device-related thrombosis. In contrast to the field of TAVI where various RCTs have helped to identify the optimal antithrombotic therapy based on patient characteristics, evidence-based recommendations are still lacking for all other types of transcatheter interventions. Available results suggest that although DRT is a relatively common and potentially severe complication, systematic use of a potent antithrombotic therapy or association of various agents may result in an intolerable bleeding risk, emphasising the need for tailored approaches for each type of transcatheter intervention and patient characteristic to identify the fine balance between safety and efficacy.
Conflict of interest statement
J-P. Collet reports support from AstraZeneca, Boston Scientific, Bristol-Myers Squibb, COR2ED, Lead-Up, Medtronic, and WebMD. J. Rodés-Cabau reports institutional research grants from Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott; and consultant/speaker fees from Edwards Lifesciences and Medtronic. J.M. Ten Berg reports grants from AstraZeneca and from the St. Antonius research fund; and personal fees from Boehringer Ingelheim, AstraZeneca, Bayer, and Ferrer. S. Windecker reports research, travel or educational grants to the institution without personal remuneration from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, B. Braun, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, Cardiovalve, Cordis Medical, CorFlow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Farapulse Inc., Fumedica, Guerbet, Idorsia, Inari Medical, InfraRedx, Janssen-Cilag, Johnson & Johnson, MedAlliance, Medicure, Medtronic, Merck Sharp & Dohme, Miracor Medical, Monarq, Novartis, Novo Nordisk, Organon, OrPha Swiss, Pharming Group N.V., Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave; and he has served as an advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, and V-Wave, with payments to the institution but no personal payments; he is also a member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. D.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, Novo Nordisk, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura; he also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, and the Scott R. MacKenzie Foundation. G. Montalescot reports during the past 2 years research grants to the institution or consulting/lecture fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, CellProthera, CSL Behring, Europa, Idorsia, IRIS-Servier, Medtronic, MSD, Novartis, Pfizer, Quantum Genomics, and Sanofi-Aventis. P. Guedeney has no conflicts of interest to declare.

