Abstract
Stroke in patients with valvular heart disease undergoing transcatheter intervention is relatively rare but associated with significant morbidity and mortality. Risks vary according to the underlying valve disease and proposed procedure. While major improvements have been made in developing and understanding the technical factors associated with intra- and peri-procedural stroke, uncertainty exists regarding the optimal antithrombotic management strategy after transcatheter heart valve intervention and the utility of routine cerebral embolic protection. Further clinical studies are warranted to minimise these complications.
Background
The overall population prevalence of valvular heart disease in industrialised countries is estimated to be 2.5%, with degenerative aortic and mitral valve disease accounting for the majority1,2. Due to the great predominance of degenerative aetiologies, this prevalence increases markedly after 65 years of age2,3. The risk of cerebrovascular events (CVE) and death in these patients remains high1. Stroke is the second leading cause of mortality worldwide, and approximately 50,000 US residents with valvular heart disease develop a stroke each year4. Large population-based studies indicate that older age, presence of atrial fibrillation and severity of the valve lesion are independent predictors of CVE among patients with valvular heart disease, the latter being strongly associated with subsequent morbidity and mortality1, and probably due to concomitant diffuse atherosclerosis, congestive heart failure or a prothrombotic state3,5.
Stroke may be defined as a sudden neurological deficit due to an acute focal injury of the central nervous system (CNS) of vascular cause4,6. From the standpoints of pathophysiology and pathological anatomy, stroke can be categorised into two broad categories according to its underlying mechanism: ischaemic and haemorrhagic stroke. Ischaemic stroke can occur after either reduction or interruption of the normal cerebrovascular circulation4,7: (i) global cerebral ischaemia, which may occur following profound reduction in global cerebral perfusion pressure (such as in cardiac arrest, shock or severe sustained hypotension); and (ii) focal cerebral ischaemia, which may occur as a result of impaired perfusion in a localised area of the CNS due to vascular occlusion secondary to embolism or atherothrombosis, or profound systemic hypotension in the presence of pre-existing cerebrovascular disease8. Haemorrhagic stroke might be primary (most commonly due to uncontrolled systemic hypertension) or secondary (i.e., haemorrhagic conversion of an ischaemic stroke)9.
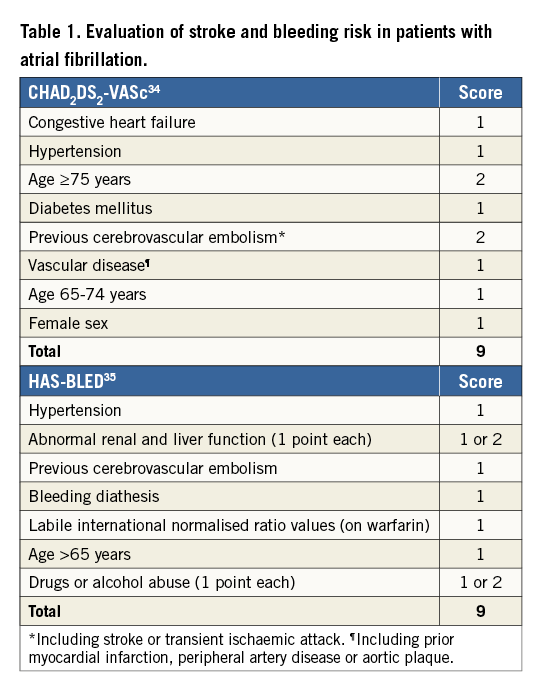
Both types of stroke might occur in patients with significant valvular heart disease, either as a part of the natural history of the valvular disorder (with or without atrial fibrillation [AF] and other cardiovascular comorbidities), or as a complication of percutaneous or surgical treatment1,2. The thromboembolic risk associated with valvular heart disease is critically amplified by the presence of concomitant AF10. Valvular AF, which can be defined as AF accompanying mitral valve disease with significant stenosis (MS) or in patients with mechanical prosthetic valves, is associated with significantly higher risk of thromboembolism compared with non-valvular AF10. AF impairs atrial contractility and thereby increases local thrombogenicity to enhance the formation of low-blood-flow red-blood-cell-rich thrombi; the major source of such thromboembolism is the left atrial appendage (LAA)11. Systemic oral anticoagulation is superior to antiplatelet therapy for stroke prevention in this setting12. Patients with a mechanical mitral valve prosthesis and AF have the highest CVE risk and require the most intense level of anticoagulation with warfarin. Subclinical (silent) AF may often precede the development of clinically overt AF and is associated with an increased risk of ischaemic stroke or systemic embolism13. Several stratification tools have been developed to individualise the risk of stroke in AF, whilst others focus on the prediction of spontaneous bleeding risk associated with chronic oral anticoagulation (Table 1). These are commonly used in clinical practice and play an important role in assessing the balance of risk and benefit in patients with valvular heart disease undergoing medical, surgical or percutaneous treatment.
Transcatheter heart valve therapies have been introduced for the treatment of acquired valvular heart disease in patients at high or extreme risk for surgical valve replacement14,15. Patients undergoing transcatheter heart valve therapies are typically old, frail and often have multiple comorbidities14,15. Given the high-risk profile of these patients and the clinical implications of cerebrovascular complications, accurate risk stratification and preventative strategies in the perioperative and mid- to long-term phase after transcatheter heart valve treatment are of paramount importance16,17.
Stroke after transcatheter aortic valve implantation
New CNS parenchymal ischaemic lesions are very commonly detected after transcatheter aortic valve implantation (TAVI) by cerebral imaging (range 66%-86%)17. These are usually multiple, distributed in both cerebral hemispheres and clinically silent18. In contrast, the current incidence of clinically manifested stroke (major or minor) after TAVI ranges from 1%-3% at 30 days and 4%-5% at one year17,18. Strokes occurring in the acute (<24 hours) phase are strongly related to technical and procedural factors (Figure 1)18, whereas those occurring later are most likely related to other pathophysiological factors, such as chronic or new-onset AF and overall atherothrombotic disease burden and related risk factors16,18. Stroke after TAVI correlates with increased major morbidity and mortality19.
A comprehensive overview of the preventive and protective strategies for TAVI-related stroke is illustrated in Figure 1. According to the timing of incidence and its underlying pathophysiology, these preventive measures can be intra- or post-procedural strategies17,20-23. Significant clinical investigation is underway on several fronts, including embolic protection systems, use or avoidance of balloon predilation (currently being tested in the Use of the Self-Expanding Medtronic CoreValve Prosthesis Without Predilation in Patients With Severely IMPaired Left-VentrIcular Ejection Fraction for TAVI Trial [SIMPLIFy TAVI; NCT01539746]) as well as procedural and mid- to long-term anticoagulation. Protocols for antithrombotic therapy are largely based on empiric consensus: the current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines include evidence level C and recommend procedural anticoagulation with unfractionated heparin (UH, target activated clotting time [ACT] 300 sec) and possible reversal with protamine at the end of the procedure20; there are no clear recommendations in the European guidelines21. Pre-loading with high-dose antiplatelet therapy is widely employed and might be beneficial in reducing intraprocedural thrombotic complications but has never been formally evaluated. Similarly, the subtle risk-benefit balance associated with a long-term antithrombotic strategy (inherent increase in haemorrhagic risk versus enhanced CVE protection) has never been adequately tested. Patients undergoing TAVI are frail and at high risk of both types of complication, and severe aortic stenosis is characterised by abnormalities in coagulation pathways and a higher propensity for spontaneous bleeding due to acquired type 2A von Willebrand syndrome, lower platelet and haemoglobin levels22.
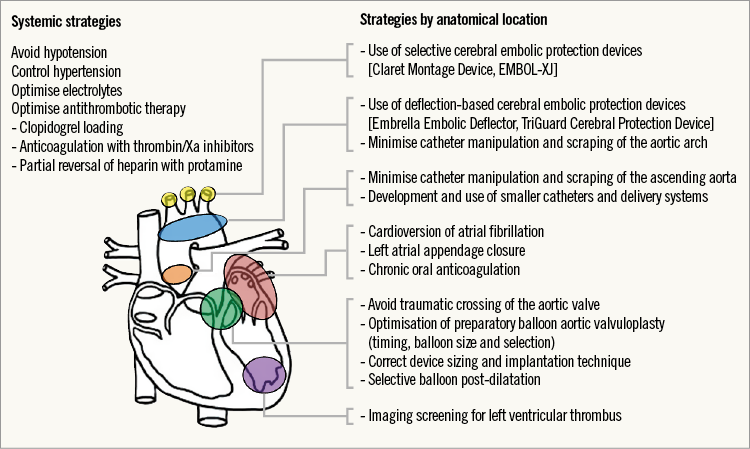
Figure 1. Strategies to prevent cerebrovascular events in patients undergoing transcatheter heart valve intervention.
Several studies have focused on the direct thrombin inhibitor, bivalirudin, for procedural anticoagulation in TAVI23-25: these are expected to provide important clinical information once completed. Post-procedural strategies for stroke prevention after TAVI focus on optimal antithrombotic management and stroke/bleeding risk stratification17,26. An overview of possible antithrombotic therapies for stroke prevention after TAVI and other modes of transcatheter heart valve replacement is illustrated in Figure 2.
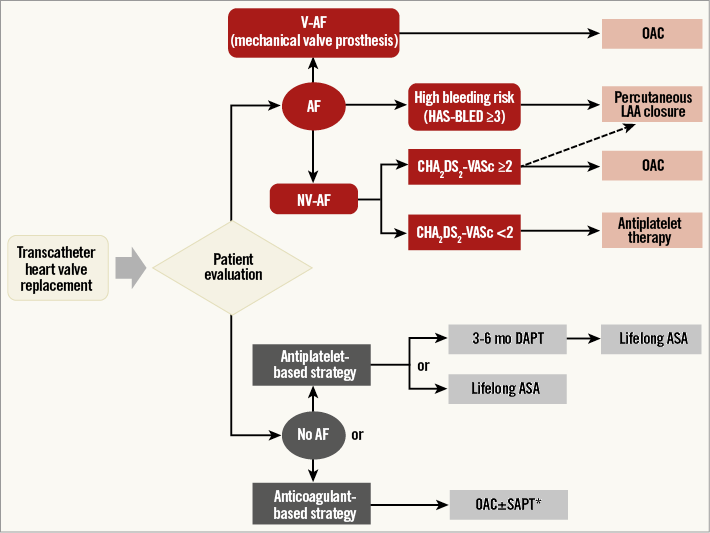
Figure 2. Potential antithrombotic treatment options for prevention of thromboembolism after transcatheter heart valve replacement. *Triple therapy (OAC+DAPT) discouraged by guidelines. AF: atrial fibrillation; ASA: acetylsalicylic acid; DAPT: dual antiplatelet therapy; LAA: left atrial appendage; OAC: oral anticoagulation; NV-AF: non-valvular atrial fibrillation; SAPT: single antiplatelet therapy; V-AF: valvular atrial fibrillation
Stroke after transcatheter mitral valve intervention
Transcatheter mitral valve interventions (TMVI) are a growing subset of percutaneous structural heart interventions aimed at treating mitral stenosis (MS) and regurgitation (MR) using minimally invasive techniques27. Both valve lesions are commonly associated with AF, which, in combination with large left atrial size and left ventricular dysfunction (even after surgical mitral valve correction) is a major determinant of cardioembolic stroke10. The impact of AF on stroke risk seems to differ in MS and MR: it is highest in MS, while pure MR may reduce the likelihood of CVE in patients with chronic AF as a result of higher flow patterns in the fibrillating atria which mitigate the risks of local stasis and ensuing hypercoagulability10. In patients with MS, most of the stroke risk might be attributable to concomitant valvular AF which is present in ≈40% of affected patients and carries an even more durable thromboembolic hazard in those with rheumatic aetiology28, even after successful treatment of the haemodynamic obstruction28. Therefore, all patients with MS in chronic AF require warfarin (target international normalised ratio 2.5-3.5); other oral anticoagulants are not approved for use in such patients28. Balloon mitral valve valvuloplasty is a well-consolidated therapeutic modality, and transcatheter valve replacement is under investigation for calcific aetiologies.
In the last few years, several devices and interventional strategies have been developed for the percutaneous treatment of MR27,29. Transcatheter edge-to-edge repair with the MitraClip (MC) system (Abbott Vascular Inc., Menlo Park, CA, USA) has been demonstrated to be safe and effective for the treatment of moderate-to-severe MR in patients at high or extreme risk for surgery29. Other devices, which target the different anatomical mechanisms underlying MR, have also been developed recently for percutaneous use27. Furthermore, several first-in-human transcatheter mitral valve replacement procedures have very recently been initiated with promising results30. Patients with mitral valve disease typically remain at high risk for CVE even after valve repair or replacement1. The risk of stroke at 30 days after MC implantation is around 1%29,31; rates of CVE at longer-term follow-up are not widely reported. Prevention of early and late CVE requires aggressive risk factor modifications, therapeutic anticoagulation and arrhythmia management.
Percutaneous left atrial appendage occlusion (LAAO) is non-inferior to OAC in patients with non-valvular AF who are at elevated risk of stroke and is superior in terms of bleeding safety and mortality. If performed at the time of percutaneous mitral or aortic valve intervention, this procedure might significantly improve long-term outcomes by reducing the risk of stroke and avoiding the risks of OAC32,33.
Conclusions
Stroke in patients with valvular heart disease undergoing transcatheter intervention is relatively rare but is associated with significant morbidity and mortality. While major improvements have been made in developing and understanding the procedural factors associated with intraprocedural and periprocedural stroke, uncertainty exists regarding the optimal antithrombotic management after transcatheter heart valve intervention and the utility of routine cerebral embolic protection. Further clinical studies are warranted to minimise these catastrophic complications.
Conflict of interest statement
The authors have no conflicts of interest to declare.

