Abstract
Transcatheter aortic valve implantation is a widely performed procedure for treatment of symptomatic severe aortic stenosis. According to the current literature, major stroke has been reported as occurring in 3-6% of patients during the first 30 days following valve implantation. Several pathological mechanisms may be involved in the development of periprocedural ischaemic stroke with the majority being due to thromboembolism and atheroembolism. One approach to reduce the incidence of procedural cerebral thromboembolic events is the use of cerebral protection devices, either deflecting (Embrella, TriGuard) or capturing (Claret, Embol-X) embolic material.
We decided to review the current evidence on this important issue focusing on the four cerebral protection devices currently available.
Introduction
Since the publication of the randomised PARTNER trials in 2010 and 2011 transcatheter aortic valve implantation (TAVI) has become a widely performed treatment for patients suffering from symptomatic severe aortic stenosis (AS). The first published PARTNER B trial established the superiority of TAVI over medical treatment in inoperable patients showing a highly significant reduction of all-cause mortality at one year (43.3% vs. 68.0%; p<0.001), paralleled by a markedly improved quality of life1. However, there was a non-significant trend towards more major strokes in the transcatheter group (5% vs. 1.1% in the medical group at 30 days; p=0.06).
In the PARTNER A trial, patients with severe AS at high risk for surgery were randomised to either transfemoral/transapical TAVI or surgical aortic valve replacement (SAVR). TAVI proved to be non-inferior as compared to SAVR with regard to all-cause mortality. Thirty-day mortality was even significantly lower in the subgroup of patients undergoing transfemoral TAVI (3.7% vs. 8.2%, as-treated analysis)2. Periprocedural and post-procedural morbidity differed considerably between the two treatment modalities: while neurologic events and vascular complications occurred more often in the TAVI group, the SAVR population suffered from more major bleedings and experienced more new onset atrial fibrillation. The rate of major strokes did not differ between the treatment groups and, after 30 months3, numerically more strokes had occurred in the SAVR population. Was the “stroke issue” overrated overall?
According to a 2012 published meta-analysis including more than 10,000 patients, the 30-day neurologic event rate was 3.3±1.8% (range 0-6%) with a 3.5-fold increase in 30-day mortality in patients experiencing a stroke (25.5±21.9% vs. 6.9±4.2%)4. Therefore, stroke prevention is an important issue.
Pathogenesis of stroke in TAVI
Cerebral imaging studies reported silent ischaemic lesions (mostly multiple) in 68-91% of patients a few days after TAVI5-7. These lesions were not associated with symptoms or measurable cognitive impairment, and 80% of the detected lesions disappeared after three months8.
Several mechanisms may be involved in the pathogenesis of periprocedural stroke in TAVI, including embolisation of calcified or atheromatous particles, thromboembolism and air embolism, as well as prolonged hypotension and haemorrhage. Histopathological analyses of fragments captured during TAVI demonstrated thrombotic material (fibrin) and fragments compatible with aortic valve leaflet or aortic wall origin (amorphous calcium, and connective tissue) in the majority of cases9.
During TAVI, procedural steps associated with manipulation of the aortic valve may cause most of the cerebral embolisms. Simple retrograde passage of the stenotic aortic valve with diagnostic catheters is associated with silent cerebral embolism in 22% of patients10. Transcranial Doppler (TCD) studies during TAVI confirmed the majority of cerebral microembolisms occurring at the time of balloon valvuloplasty, deployment and positioning of the prosthetic valve11,12. This may explain why intraprocedural neurologic events are not more common in transfemoral procedures as compared to transapical TAVI, despite the theoretical advantage of sparing the aortic arch when a transapical approach is chosen6,13.
Only 40-50% of symptomatic strokes occur within 24 hours after TAVI14,15, resulting in more than half of the strokes not being directly procedure-related.
Established risk factors for early stroke include small aortic valve area, prior history of cerebrovascular accidents, balloon post-dilatation, multiple valve implantation attempts, and (new onset) atrial fibrillation14-17. The incidence of stroke continues to be high up to 60 days after valve implantation, followed by a low but constant risk during long-term follow-up, mainly influenced by individual risk factors (e.g., BMI <20 kg/m2, age >80 years, chronic atrial fibrillation and peripheral and cerebrovascular disease)17,18.
Requirements for cerebral protection devices
The goal of a cerebral protection device is to prevent clinically relevant intraprocedural strokes. As stroke is a rare complication, silent new cerebral lesions as assessed by diffusion-weighted MRI (DW-MRI) or high-intensity transient signals (HITS) detected by transcranial Doppler are often used as endpoints in studies. The clinical meaning of new perfusion defects, however, remains a matter of controversy. According to surgical literature, the lesion “load” detected by MRI has been associated with postoperative cognitive decline19, a relation that has not been confirmed in patients undergoing TAVI8,20.
Evidence from carotid stenting and open heart surgery suggests that mechanical cerebral protection has the potential to reduce stroke rate and to improve clinical outcome21,22.
Despite these theoretical advantages when using cerebral protection devices, positioning of the device in the aortic arch itself carries a certain risk of mobilising atheromatous material and causing damage to the arterial wall, possibly resulting in a neurologic event. This makes an excellent safety profile and the use of antithrombogenic material the most important requirements for cerebral protection devices. Furthermore, protection devices are expected to warrant stable position, to fit anatomical variations of the aortic arch, to allow sufficient perfusion of the protected arterial territories, and to avoid interference with valve delivery. Deployment has to be simple and fast in order not to lengthen and complicate the TAVI procedure. There are currently four devices in investigational use potentially meeting these requirements (Table 1).
Currently available cerebral protection devices
EMBOLIC DEFLECTION DEVICES
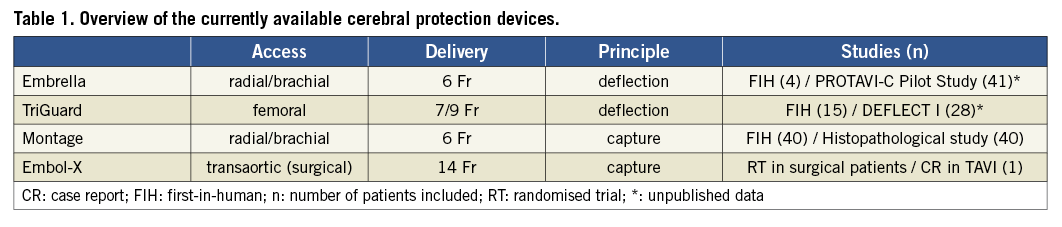
The Embrella device (Edwards Lifesciences, Irvine, CA, USA), introduced via radial or brachial arterial access, deflects emboli from the innominate and the left carotid arteries. In 60% of patients the device additionally covers the left subclavian artery. The device consists of two hydrophilic heparin-coated polyurethane membranes with 100 μm pores. An oval umbrella-like nitinol frame holds the membranes and is attached to a 0.035” (0.89 mm) nitinol cable (Figure 1). After introduction of a 6 Fr long delivery sheath through the radial or brachial artery to the aortic arch, the Embrella device is advanced under fluoroscopic guidance, exiting the sheath in the aortic arch, where the two petals of the umbrella are stabilised at the outer curvature (Figure 2).
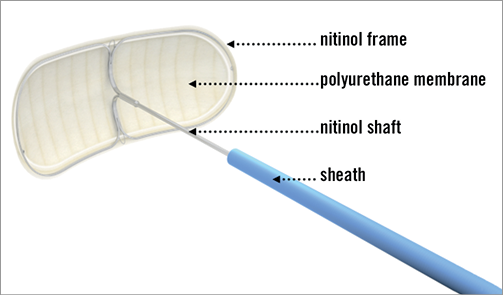
Figure 1. The Embrella device consists of an umbrella-like nitinol frame holding two polyurethane membranes (courtesy of Edwards Lifesciences, Irvine, CA, USA).
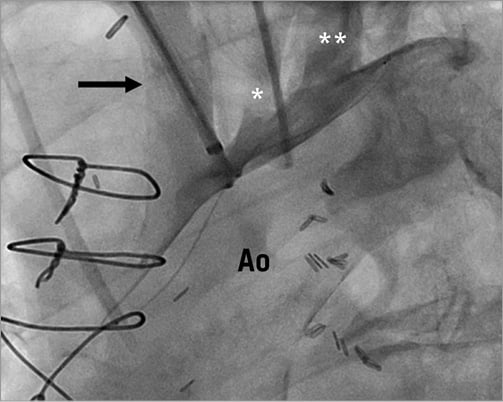
Figure 2. Embrella device positioned at the inner curvature of the aortic arch. The device fully covers the brachiocephalic trunk (arrow), the left carotid artery (*), and the left subclavian artery (**). Ao: aorta
A 2010 published first-in-human study in four patients confirmed feasibility of the device use23. Currently, a double randomised trial is ongoing in Europe and Canada (PROTAVI-C for Prospective Randomized Outcome study in patients undergoing TAVI to Examine Cerebral Ischemia and Bleeding Complications). The study aims to investigate the efficacy of the Embrella device for prevention of new intraprocedural cerebral lesions (assessed by diffusion-weighted MRI) in 500 patients. In a second post-procedural phase, the patients will be randomly allocated to either single or dual antiplatelet therapy and followed for one year. The results of the PROTAVI-C Pilot Study were presented at EuroPCR 2013: in 41 patients the Embrella device was successfully deployed and retracted. One patient suffered a non-device-related minor stroke on day two. On DW-MRI, all (100%) patients showed new ischaemic lesions. However, the lesion volume was markedly smaller as compared to patients where no cerebral protection was used. Intraprocedural transcranial Doppler showed most HITS during valve crossing and prosthesis positioning, closely followed by a high number of HITS during deployment of the device.
The TriGuardTM Cerebral Protection Device (Keystone Heart Ltd, formerly SMT Research & Development, Caesarea, Israel) is introduced via the femoral artery. The concept is similar to that of the Embrella device with some notable differences. A 9 Fr sheath is usually used for delivery and retrieval and allows additional placement of a pigtail catheter for procedural guidance. The device itself consists of a nitinol mesh and a nitinol frame with two stabilisers that anchor the device in the brachiocephalic trunk and at the inner curvature of the aortic arch (Figure 3). The system was first designed as a permanent surgical implant for cerebral protection in patients at high risk for recurrent embolisms.
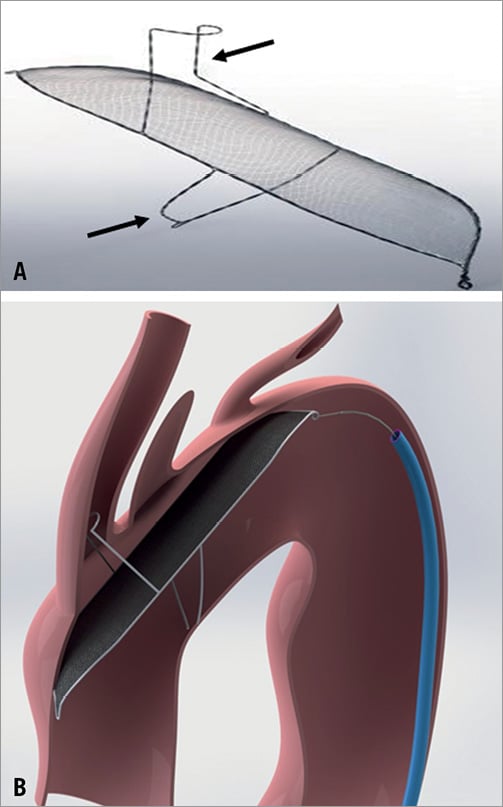
Figure 3. A) The TriGuard Cerebral Protection Device consists of a porous membrane and a nitinol frame with two stabilisers (arrows) (courtesy of Keystone Heart Ltd, Caesarea, Israel). B) Device anchored in the brachiocephalic trunk and at the inner curvature of the aortic arch. The design allows coverage of all three neck vessels (courtesy of Keystone Heart Ltd, Caesarea, Israel).
Initial experience in 15 patients proved feasibility24 of the device use. At EuroPCR 2013 preliminary results from the prospective DEFLECT I trial were presented: initial device positioning was successful in 100% of patients. However, after removal of the TAVI delivery system, only 79% of devices were still correctly positioned and only 68% of devices still covered all three neck vessels. Of the 28 study patients, none had adverse clinical events associated with the device use, while two suffered a non-device-related stroke on day one after TAVI. The total number of patients with new ischaemic cerebral lesions on DW-MRI was not reduced significantly as compared to historical cohorts where no cerebral protection device was used (70% vs. 76%), while the total volume of new lesions was reduced in patients where the TriGuard was used (1.64 cm3 vs. 0.70 cm3).
EMBOLIC CAPTURE DEVICES
The MontageTM Dual Filter System (Claret Medical Inc., Santa Rosa, CA, USA) is designed to capture embolic debris travelling to the brain in the brachiocephalic trunk and the left common carotid arteries (Figure 4). The catheter is delivered through a 6 Fr sheath via the radial or brachial artery. The conically shaped filters consist of a nitinol frame and polyurethane laser-drilled filter membrane with 140 μm diameter pores. The filter frames are radiopaque and once deployed seal against the vessel wall, allowing filtered blood to pass to the brain while trapping debris. After positioning of the first filter in the brachiocephalic trunk, the catheter is advanced further in the aortic arch under fluoroscopic guidance and the tip of the delivery system is curved towards the left common carotid artery for placement of the second filter. Anatomical requirements are a diameter of the brachiocephalic trunk measuring between 9 and 16 mm and a left common carotid artery diameter between 6 and 10 mm. Recent improvements over the first-generation device include compatibility with 0.014” standard coronary guidewires, a modified curve of the device tip to simplify antegrade intubation of the left carotid artery, hydro coating, and implementation of an ergonomic handle. The safe use of the system has been demonstrated in a 2012 published first-in-man study, which included 40 patients (seven patients received the first-generation device and 33 patients the second-generation device). Successful placement of both filters was achieved in 60% of patients with the first-generation device and in 87% of patients with the second-generation device. Embolic debris was collected in 54% of patients at termination of the TAVI procedure. Complications directly related to device insertion occurred in four patients (10%) requiring surgery in three patients (7.5%) (one dissection of the radial artery and two brachial pseudoaneurysms). Early stroke (<24 hr) was reported in one patient (2.5%)25. In another study exclusively using the second-generation device, technical success was 100% without any device-related complications. Macroscopic debris was captured in at least one of the two filters in 75% of the cases9. To date, no data exist on the potential reduction of cerebral embolisms when using the device (e.g., by means of reduction of HITS on TCD or new ischaemic lesions on DW-MRI). A larger, multicentre, randomised trial filling this gap is currently pending.
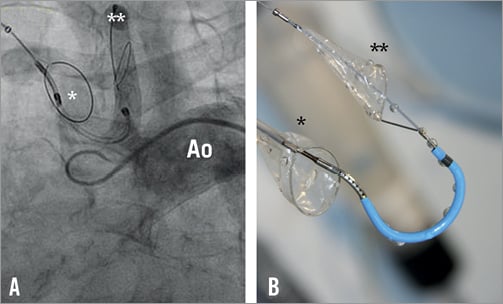
Figure 4. A) Angiographic view of the Montage Dual Filter System successfully positioned in the brachiocephalic trunk (*) and the left carotid artery (**) (courtesy of Claret Medical Inc., Santa Rosa, CA, USA). B) The device consists of two conically shaped polyurethane filters and a nitinol frame. The proximal filter (*) is deployed in the innominate artery and the distal filter in the left carotid artery (**) (courtesy of Claret Medical Inc., Santa Rosa, CA, USA). Ao: aorta
The Embol-X intra-aortic filter (Edwards Lifesciences, Irvine, CA, USA) was designed for surgical use. In surgical patients the device is introduced via a sidearm of a modified 24 Fr intra-aortic cannula and deployed in the ascending aorta before removal of the aortic clamp (Figure 5). It remains in place as long as the patient is on cardiopulmonary bypass. The filter consists of a polyester mesh capturing debris >120 µm and is currently available in five different sizes fitting internal aortic diameters of 2.2 to 4.0 cm. In a randomised trial including almost 1,300 patients undergoing open heart surgery the device demonstrated capture of debris in up to 97% of patients. No particular safety concerns were raised. However, no significant differences in the rate of apparent strokes between the two study groups (2.6% in the filter group vs. 2.2% in the control group; p=0.59) were seen. The occurrence of silent cerebral lesions has not been investigated. Overall, better outcome was observed in patients with a low body mass index (<25 kg/m2), low ejection fraction (≤25%) or previous history of cerebrovascular disease. These differences were mainly driven by a reduction in postoperative renal failure in the filter group22.
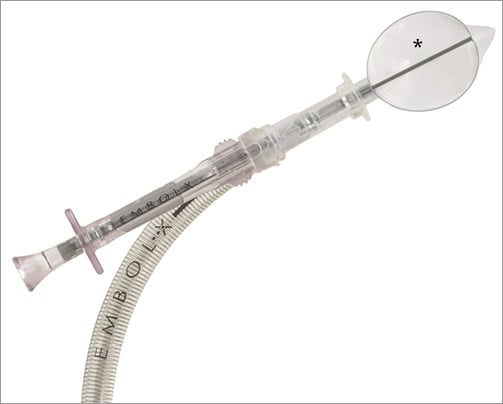
Figure 5. The Embol-X device is introduced via a sidearm of a modified 24 Fr intra-aortic cannula. The polyester mesh (*) captures debris with a diameter >120 µm.
In TAVI, the device can exclusively be introduced through an upper mini-sternotomy providing direct visualisation of the ascending aorta. In 2011, the filter was successfully deployed over a 14 Fr sheath in one patient during transaortic TAVI26. At follow-up (48 hr) the patient developed no silent or apparent cerebral complication. Since the transaortic access is the only TAVI access allowing insertion of the Embol-X device, its use in the current design will remain limited in TAVI.
Conclusions
Prevention of stroke is an important issue to improve outcome in TAVI further. This is particularly true in the light of expansion of TAVI from high-risk to intermediate-risk patients. As an established risk factor for the occurrence of early post-procedural neurological events, new onset atrial fibrillation requires timely diagnosis and adequate treatment. Left atrial appendage closure is a very attractive alternative to oral anticoagulation in these patients27,28.
Furthermore, smaller delivery catheters have the potential to reduce the stroke rate even more29,30. If a self-expandable valve prosthesis is used, avoiding balloon aortic valvuloplasty eliminates an important source of microembolism during TAVI31. The development of a true percutaneous transapical approach with percutaneous apical closure32 may be less traumatic and improve outcome of transapical TAVI.
It is of note that only about 50% of the cerebrovascular events observed during the 30-day period following TAVI are believed to occur during the procedure itself while the remainder of strokes occur weeks to months after implantation. As a consequence, defining the most appropriate antiplatelet or anticoagulation regimen and its duration following TAVI may have a significant impact on stroke rate, which makes medical therapy an important pillar of stroke prevention after TAVI. Mechanical cerebral protection has the theoretical potential to reduce procedural stroke rate at most by 50%. Besides local complications at the insertion site, results from the PROTAVI-C Pilot Study confirm that placement of the device itself carries a risk of causing cerebral embolism. From preliminary results of the DEFLECT I trial, we learn that there is a risk of device interference with the valve delivery system. Whether a reduction in lesion volume justifies the risk of causing potential harm will hopefully be answered at termination of the studies.
At this stage, broad use of cerebral protection devices during TAVI cannot be recommended and their use should –outside of a clinical trial– be restricted to patients at very high risk for embolic events (e.g., patients with mobile structures on the aortic valve).
Conflict of interest statement
F. Nietlispach is a consultant and proctor to Edwards Lifesciences, Irvine, CA, USA. F. Praz has no conflicts of interest to declare.

