Transcatheter aortic valve implantation (TAVI) has evolved as an efficient and safe treatment of severe aortic stenosis even in patients at high surgical risk. Although the rate of stroke with TAVI has decreased over time, this is still a feared and devastating complication. Manipulation of the transcatheter heart valve system across the aortic arch and within the aortic root may lead to embolisation of debris to the brain. Thus, even in the absence of clinical symptoms, most patients have new diffusion-weighted magnetic resonance perfusion brain defects after TAVI. Cerebral embolic protection (CEP) systems have been developed to reduce the risk of procedural stroke. The CEP systems can be divided into 2 groups: deflection devices which aim to send debris “anywhere but the brain”, and filter devices which capture debris en route to the brain. The most commonly used device is the SENTINEL CEP (Boston Scientific), which is introduced via a 6 Fr right radial artery sheath and consists of 2 filters: 1 in the proximal part of the brachiocephalic artery and 1 in the left common carotid artery. These filters provide protection for 80-90% of the blood flow to the brain, only leaving the area supplied by the left vertebral artery unprotected. Prior small, randomised trials have demonstrated that the SENTINEL device is safe and successfully captured embolic debris in almost all patients, but the primary endpoint of a reduction in new cerebral lesion volume was not statistically significant. Importantly, these trials were not powered to assess a reduction in clinical stroke with the SENTINEL system123. Such inconclusive data have led to variable penetration of CEP use during TAVI with only a few sites using it routinely, whereas more sites consider SENTINEL for patients at high risk for stroke, e.g., severely calcified aortic valve, bicuspid aortic valves, valve-in-valve procedures, and prior stroke. Large-scale randomised clinical trials powered for clinical endpoints to determine the role of CEP have been eagerly awaited.
The results of the PROTECTED TAVR (Stroke PROTECTion With SEntinel During Transcatheter Aortic Valve Replacement) Trial (ClinicalTrials.gov: NCT04149535) were presented recently4; this pragmatic trial randomised 3,000 patients to TAVI using the SENTINEL CEP device (n=1,501) or TAVI without CEP (n=1,499). The primary endpoint was stroke at hospital discharge or 72 hours, with mandatory neurology assessment before and after the TAVI procedure. Despite a relative 19.2% reduction in the primary endpoint, there was no statistically significant difference in stroke rates between the TAVI+CEP and TAVI-only groups, with 34 (2.3%) and 42 (2.8%) events in each group, respectively (Figure 1). Keeping in mind that the primary endpoint was not met and, hence, there was a need for caution in assessing secondary endpoints, a prespecified analysis of disabling stroke revealed a 60% relative risk reduction for TAVI+CEP as compared to TAVI only, with 8 (0.5%) vs 20 (1.3%) events (p=0.0225), respectively (Figure 1). Despite the uncertainty in the efficacy of the device, the safety of SENTINEL was confirmed, with only one patient (0.1%) experiencing a CEP-related access-site vascular complication.
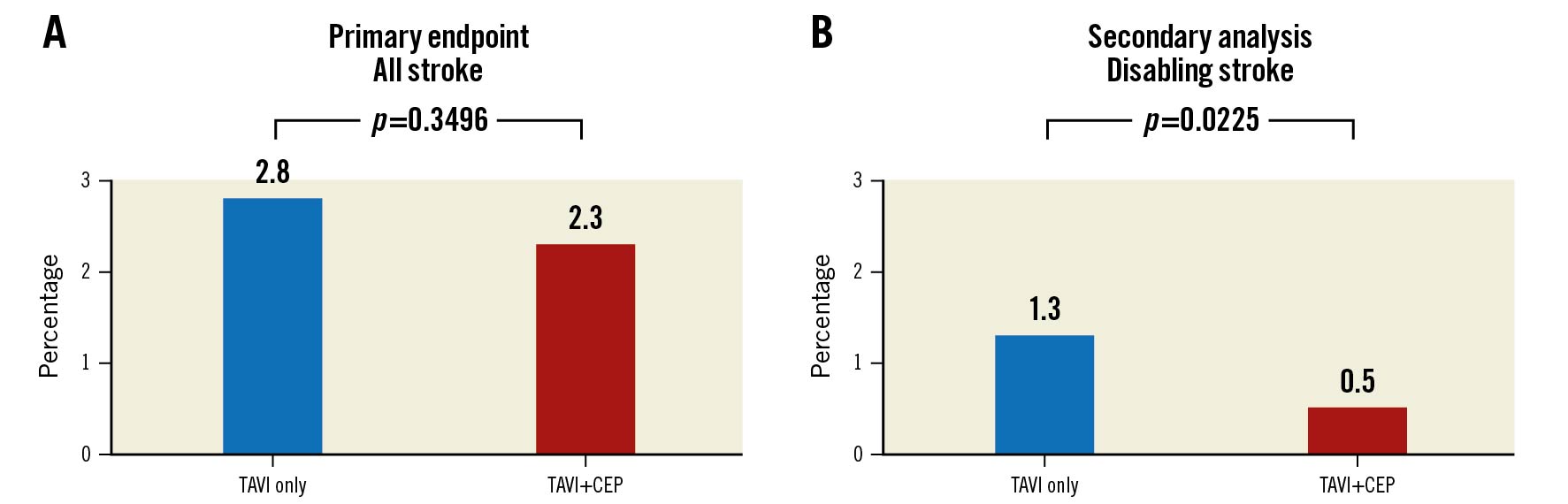
Figure 1. Primary endpoint and secondary analysis. A) Primary endpoint of all strokes through to 72 hours and B) secondary analysis of disabling strokes through to 72 hours for TAVI only or TAVI with CEP. CEP: cerebral embolic protection; TAVI: transcatheter aortic valve implantation
How should these results be interpreted?
Firstly, the stroke rate in the control group of the PROTECTED TAVR trial was lower than anticipated. The trial was designed with an anticipated stroke rate of 4% in the TAVI-only arm and with an estimated 50% reduction to 2% in the TAVI+CEP arm. It is uncertain why the stroke rate was lower than expected in a study population with a mean age close to 80 years, a mean Society of Thoracic Surgeons (STS) score of 3.4%, and where nearly one-third of the patients were deemed to be at high or extreme surgical risk by the Heart Team. One potential explanation could be patient selection bias, where some sites used CEP in patients considered at high risk for stroke and, thereby, only enrolled patients at lower risk in the trial. Such practice was feasible since the device is commercially available in the USA and the European Union. However, bicuspid aortic valves and valve-in-valve procedures accounted for 8% and 3%, respectively, of the patients in the PROTECTED TAVR trial. It is unclear if the rates of stroke and the efficacy of the device would be increased in a higher-risk patient population.
Secondly, although the endpoint of overt stroke is logical and is the fundamental goal of CEP, the long-term consequences of ubiquitous ‘silent’ brain lesions may also be important. It is likely that these lesions contribute to cognitive impairment and early dementia, important endpoints that become ever more relevant as TAVI expands to even younger patients with a longer life expectancy. Unfortunately, the PROTECTED TAVR trial did not capture data on silent lesion numbers with diffusion-weighted magnetic resonance imaging and will not evaluate long-term cognitive outcomes. Thus, we should not infer that the SENTINEL device impacts these endpoints.
Thirdly, subgroup analyses did not identify any specific populations which might benefit from CEP or any procedural factors potentially associated with a high risk of stroke. This is unfortunate, as this information would be very useful to clinicians and patients alike. Looking for predictors of stroke is statistically challenging in the context of a relatively small number of events.
Finally, it may be argued that the statistically significant reduction of the important secondary endpoint, disabling stroke, is more meaningful for the patient and the healthcare system than non-disabling stroke. Without disregarding the impact for the individual patient facing this devastating complication and the peril of overinterpreting secondary analyses, in a trial with a negative primary endpoint, a cost-benefit analysis would be of utmost interest, since the number needed to treat (to prevent 1 disabling stroke event) is 125.
How will the results of the PROTECTED TAVR trial influence our daily clinical practice?
Currently, the penetration of CEP during TAVI in the European Union and the USA is approximately 5% and 15%, respectively. These numbers are likely to fall based on the results of the PROTECTED TAVR trial, though it will be interesting to see how use of the SENTINEL device develops among perceived high-risk patients in institutions where reimbursement is in place. Despite the clear evidence of safety, it will be difficult to justify the cost of using these systems in the absence of clear efficacy data.
Addition trial evidence that will ultimately determine the use of the SENTINEL device will be provided by the British Heart Foundation (BHF) PROTECT-TAVI (Prospective Randomized Outcome Study in TAVI Patients Undergoing Periprocedural Embolic Cerebral Protection With the Sentinel™ Device) trial (ClinicalTrials.gov: NCT02895737). This study which will randomise 7,730 patients in a 1:1 ratio to receive TAVI+SENTINEL or TAVI only with a primary endpoint of clinical stroke (no pre/post-TAVI neurology assessment) 72 hours post-TAVI. Since CEP is not reimbursed in the UK, the PROTECT-TAVI trial will probably include the highest-risk patients undergoing TAVI and eliminate the selection bias that may have influenced the PROTECTED TAVR trial. Moreover, the absence of a formal neurological assessment prior to and after TAVI and, hence, the focus on “clinically apparent” stroke is a real strength of this study that necessitates the inclusion of more than twice the number of patients as included in the PROTECTED TAVR trial. The PROTECT-TAVI trial includes nearly all TAVI sites in the UK and has so far enrolled more than one-third of the required subjects. A meta-analysis of the PROTECTED TAVR and the BHF PROTECT-TAVI trials is planned and will include more than 10,000 patients to guide the future of SENTINEL use in TAVI.
Finally, it is important to consider that the design limitations of the SENTINEL device itself (only 80-90% brain coverage) could be responsible for the negative results of the PROTECTED TAVR trial. A variety of alternate CEP devices, which potentially provide complete brain protection, are currently in clinical trials. It is important that these CEP device trials endure and that new stroke prevention strategies continue to emerge as we look to mitigate the devastating impact of stroke in patients undergoing TAVI.
Conflict of interest statement
L. Sondergaard has received consultant fees and institutional research grants from Boston Scientific. D. Mylotte is a consultant for Medtronic, Microport, and Boston Scientific. D. Capodanno has no relevant conflicts of interests to declare.
Supplementary data
To read the full content of this article, please download the PDF.

