Abstract
Transcatheter aortic valve implantation (TAVI) is an established treatment for aortic stenosis. Cerebral embolic protection (CEP) devices may impact periprocedural stroke by capturing debris destined for the brain. However, there is a lack of high-quality randomised trial evidence supporting the use of CEP during TAVI. The British Heart Foundation (BHF) PROTECT-TAVI trial will address whether the routine use of CEP reduces the incidence of stroke in patients undergoing TAVI. BHF PROTECT-TAVI is a prospective, open-label, outcome-adjudicated, multicentre randomised controlled trial. The trial is open to all adult patients scheduled for TAVI at participating specialist cardiac centres across the United Kingdom who are able to receive the CEP device. The trial will recruit 7,730 participants. Participants will be randomised in a 1:1 ratio to undergo TAVI with CEP or TAVI without CEP (standard of care). The primary outcome is the incidence of stroke at 72 hours post-TAVI. Key secondary outcomes include the incidence of stroke and all-cause mortality up to 12 months post-TAVI, disability and cognitive outcomes, stroke severity, access site complications and a health economics analysis. The sample size of 7,730 participants has 80% power to detect a 33% relative risk reduction from a 3% incidence of the primary outcome in the controls. Trial recruitment commenced in October 2020. As of October 2022, 3,068 patients have been enrolled. BHF PROTECT-TAVI is designed to provide definitive evidence on the clinical efficacy and cost-effectiveness of using routine CEP with the SENTINEL device to reduce stroke in TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) is an important treatment option for aortic stenosis (AS), with a robust evidence base supporting its safety and effectiveness. The risks of TAVI include vascular access site complications, stroke and death12.
Stroke is an unpredictable complication associated with TAVI which typically occurs within 72 hours of the procedure and leads to a prolonged hospital stay, a reduced chance of returning to independence, and a near 6-fold increased risk of death within 30 days3456. Stroke increases the cost of the index hospitalisation and doubles rehospitalisation costs7. It will become an even more important concern to patients and healthcare funders as TAVI is used for younger patients, and in greater numbers as the proportion of older people increases in the population. Reducing the risk of stroke during TAVI has important implications for improving patient outcomes and reducing the burden on limited healthcare resources.
Cerebral embolic protection (CEP) devices are designed to capture debris released during TAVI and prevent this from reaching the brain. The evidence supporting the use of CEP in TAVI is from (i) proof-of-principle studies which have confirmed that debris is retrieved from CEP devices, (ii) imaging studies using magnetic resonance imaging scanning to identify brain injury have confirmed that the use of CEP devices was associated with a reduced volume of new lesions8, and (iii) clinical evidence from small-scale randomised trials, powered for brain imaging surrogate endpoints, clinical case series910, systematic reviews1112, and meta-analysis studies13.
However, high-quality evidence from adequately powered randomised controlled trials is needed to establish the safety, efficacy and cost-effectiveness of the technique and guide best practice in this important and expanding clinical field. Indeed, the UK National Institute for Health and Care Excellence interventional procedure guidance committee (IPG 650) stated that while there are no major safety concerns over the use of CEP devices, the evidence for clinical efficacy is inconclusive14. In the Stroke Protection With Sentinel During Transcatheter Aortic Valve Replacement – PROTECTED TAVR Trial − (ClinicalTrials.gov: NCT04149535), 3,000 patients were randomised to standard care or use of the SENTINEL (Boston Scientific) device15. The control group had a stroke rate of 2.9% and the SENTINEL group 2.3%. This failed to reach statistical significance, as the trial was powered for an event rate of 4% in the control group and 2% in the SENTINEL group. This trial is therefore underpowered, and larger trials are needed. There were no safety concerns raised about the use of SENTINEL in this trial.
The BHF PROTECT-TAVI trial will address the question of whether the routine use of CEP in TAVI reduces the incidence of clinical stroke in patients undergoing TAVI. This is an important study because it is powered on a single clinically relevant outcome of stroke in an unselected national TAVI population and will complement the results from PROTECTED TAVR.
STUDY DESIGN
BHF PROTECT-TAVI is a prospective, open-label, outcome-adjudicated, multicentre randomised controlled trial evaluating the use of a CEP device in participants with aortic valve stenosis scheduled for treatment by TAVI.
SETTING
The trial will recruit 7,730 participants receiving TAVI from specialist cardiac centres across the UK.
STUDY POPULATION, INCLUSION AND EXCLUSION CRITERIA
The target population are patients with severe AS who are scheduled for TAVI. The BHF PROTECT-TAVI trial is designed with broad inclusion criteria and no specific exclusion criteria.
INCLUSION CRITERIA
– Participant is willing and able to give informed consent for participation in the trial.
– Aged 18 years or above.
– Considered to be candidates for TAVI by the clinical team (via any access route where CEP may be used) .
– Participant is suitable for treatment with the CEP device in the opinion of the treating physician.
EXCLUSION CRITERIA
There are no specific exclusion criteria.
Participant enrolment
Screening and eligibility assessment
Participating centres will consider all patients who are scheduled for TAVI. Screening will take place prior to consent and randomisation. Current or previous participation in randomised trials will not be disqualifying unless treatment is expected to impact on the primary outcome of BHF PROTECT-TAVI.
Informed consent
Participants must provide written consent before the TAVI procedure. Patients lacking the capacity to consent are not eligible for BHF PROTECT-TAVI. Witnessed, written informed consent with a date and signature is collected.
Randomisation
Randomisation is performed via a web-based randomisation system (Sealed Envelope Ltd, London, UK). Randomisation must be performed before the start of the TAVI procedure. Randomisation is carried out in a 1:1 ratio to either arm using randomly permuted blocks, stratified by centre.
Intervention arm
Participants randomised to the intervention arm will receive CEP during their TAVI.
Intervention details
The SENTINEL dual-filter device is a single-use, embolic protection catheter, currently approved for use in Europe and the USA. The device is inserted into the right radial or brachial artery and employs two filters (nitinol frames with 140 micron pore polyurethane film), one delivered to the brachiocephalic artery (proximal filter), and one to the left common carotid artery (distal filter) before TAVI. The SENTINEL dual-filter device will be used in accordance with its European Conformity (CE) marking.
Control arm
Participants in the control arm will receive standard of care TAVI without the use of CEP.
Outcomes
Primary outcome
The primary outcome is stroke at 72 hours (or at hospital discharge, if sooner).
Stroke is defined as a new or worsened focal or global neurological deficit of presumed vascular origin, either ischaemic or haemorrhagic, occurring after randomisation and persisting for longer than 24 hours or leading to death. In this definition, a new stroke will not be defined exclusively by brain imaging, and a clinical deficit must be present for longer than 24 hours16. Patients felt to have had a stroke by local investigators based on a clinical deficit less than 24 hours in duration, but with imaging evidence of infarction in the relevant vascular territory, will be included as part of the secondary outcome analysis. The definition of stroke will include patients who have identified occlusion of the cerebral vessels and undergo mechanical thrombectomy within the 72-hour period after TAVI. This additional definition of stroke will allow the capture, for the primary outcome, of a small number of patients that have a complete neurological recovery as a result of that mechanical thrombectomy and, therefore, will not meet the first definition.
In keeping with other large clinical trials, stroke outcome ascertainment will be maximised by the use of the validated 8-item interview, the Questionnaire to Verify Stroke Free Status (QVSFS) on a daily basis in the 72 hours following the procedure, in addition to routine clinical review171819. An answer of “yes” on the QVSFS after the procedure will prompt a local outcome assessment using the stroke definition as described above. The clinical diagnosis of stroke will be defined by local pathways, including the stroke team if appropriate. In this trial we have not mandated neurological clinical assessment before and after the procedure. However, where stroke is suspected, involvement of stroke teams is expected.
Secondary outcomes
We will analyse the following prespecified secondary outcomes:
1. Combined incidence of all-cause mortality or stroke (as defined) at 72 hours post-TAVI or hospital discharge (if sooner).
2. Incidence of all-cause mortality at 72 hours and at 12 months.
3. Incidence of stroke, as defined by centrally held National Health Service (NHS) data, between 72 hours post-TAVI (or discharge from hospital, if sooner) up to 30 days post-TAVI.
4. Incidence of stroke, as defined by centrally held NHS data, between 30 days post-TAVI up to the end of the study.
5. Stroke severity assessment using the National Institutes of Health Stroke Scale in participants who have a stroke within 72 hours post-TAVI or hospital discharge (if sooner).
6. Cognitive outcomes, as assessed using the standardised Montreal Cognitive Assessment for mild cognitive impairment at baseline, 72 hours post-TAVI (or hospital discharge if sooner), 6-8 weeks post-TAVI and 12 months post-TAVI.
7. Disability outcomes, as assessed using the simple modified Rankin Scale questionnaire at discharge, 6-8 weeks post-TAVI and at 12 months post-TAVI for participants who have a stroke within 72 hours post-TAVI or hospital discharge (if sooner).
8. Incidence of access site vascular complications, assessed according to standard criteria defined by the Valve Academic Research Consortium (VARC)-220, at 72 hours post-TAVI (or hospital discharge if sooner) and 6-8 weeks post-TAVI.
9. Cost-effectiveness analysis: data on quality of life and resource utilisation will be collected for a formal cost-effectiveness analysis (Supplementary Appendix 2). The validated EQ-5D-5L questionnaire will be used to assess quality of life at baseline, 6-8 weeks post-TAVI and at 12 months post-TAVI.
Outcome adjudication
The stroke events will be adjudicated by an independent clinical events committee (CEC) using a standard protocol to limit bias. The CEC will be blinded to trial treatment.
Sample size calculation
Assuming the proportion experiencing the primary outcome is 3% in the control arm, we will require 7,652 patients to detect a 33% relative risk reduction (risk ratio 0.67) to 2%. Assuming 1% losses/withdrawals, we will recruit 7,730 patients, for 80% power and 5% significance. A trial of this size also provides the power to give strong evidence on the effect of CEP on the secondary outcomes. For example, for a 33% relative reduction in the secondary combined outcome of all-cause mortality or stroke at 72 hours from a combined rate of 4.2%, a trial of 7,730 would provide power well in excess of 90%. Please see the Supplementary Appendix 3 for further details on the sample size calculations.
Trial procedures
Following eligibility screening and consent, a baseline assessment will be conducted prior to the TAVI procedure. Following their TAVI procedure, patients will be assessed daily for 72 hours (or up to discharge, if sooner). Post-discharge, patients will be contacted at 6-8 weeks and 12 months post-TAVI (Figure 1, Table 1, Supplementary Appendix 4).
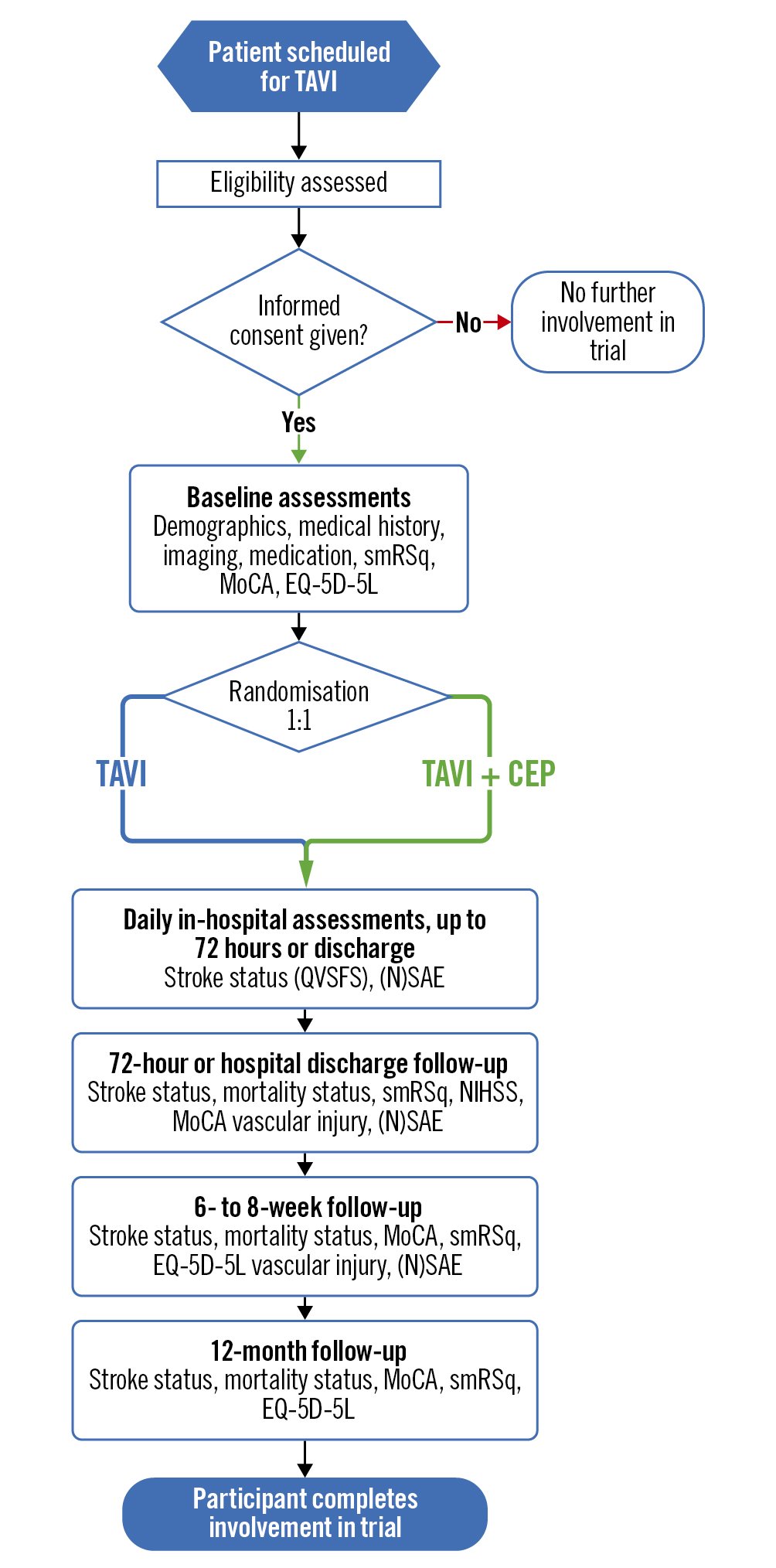
Figure 1. Flowchart showing participant progression and follow-up points for BHF PROTECT-TAVI. CEP: cerebral embolic protection; EQ-5D-5L: EuroQol’s self-assessed, 5-level, quality-of-life questionnaire; MoCA: Montreal Cognitive Assessment; NSAE: non-serious adverse event; NIHSS: National Institute of Health Stroke Score; SAE: serious adverse event; QVSFS: Questionnaire to Verify Stroke Free Status; smRSq: simple modified Rankin Scale questionnaire; TAVI: transcatheter aortic valve implantation
Table 1. Schedule of events for participants in the BHF PROTECT-TAVI trial.
| Initial patient approach | Informed consent and baseline assessments | TAVI procedure | In-hospital follow-up* | Post-discharge follow-up (in-person or remote) | ||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | 72 hours post-TAVI or discharge if sooner | 6-8 weeks post-TAVI | 12 months post-TAVI | |||
| Eligibility assessment | X | |||||||
| Informed consent | X | |||||||
| Baseline assessments | X | |||||||
| Randomisation | X | |||||||
| TAVI with CEP (intervention arm) OR TAVI without CEP (standard-of-care arm) | X | |||||||
| Stroke assessment | X | X | X | |||||
| Questionnaire to Verify Stroke Free Status (QVSFS) | X | X | X | |||||
| National Institute of Health Stroke Score (NIHSS)** | X | |||||||
| Simple modified Rankin Scale questionnaire (smRSq)** | X | X | X | X | ||||
| Stroke physician assessment** | X | X | X | |||||
| Mortality status | X | X | X | |||||
| Montreal Cognitive Assessment (MoCA) | X | X | X | X | ||||
| Vascular access site injury | X | X | ||||||
| EQ-5D-5L | X | X | X | |||||
| Adverse event reporting | X | X | X | X | X | |||
| API Study questionnaire*** | X | |||||||
| *During the patient’s in-hospital stay the QVSFS will be administered daily up to and including 72 hours or discharge from hospital, whichever is earlier. **Only to be administered if the participant’s QVSFS indicates the possible presence of a stroke. ***Only for BHF PROTECT-TAVI participants who consent to take part in the API Study. API: Assessing the quality of patient information in the BHF PROTECT-TAVI trial; CEP: cerebral embolic protection; EQ-5D-5L: EuroQol’s self-assessed, 5-level, quality-of-life questionnaire; TAVI: transcatheter aortic valve implantation | ||||||||
Adverse event reporting
Unexpected serious and non-serious adverse events will be assessed by the local primary investigator for causality and classified as follows: unrelated, unlikely to be related, possibly related, or probably related. Only those classified as unlikely, possibly or probably related will be reported to the clinical trials unit. The chief investigator will be responsible for the prompt notification of serious unexpected and related adverse events. Expected complications of TAVI and CEP are defined in the case report form for BHF PROTECT-TAVI and are not required to be reported separately.
Statistical analysis
Statistical analysis will be coordinated from the Clinical Trials Unit at the London School of Hygiene and Tropical Medicine (LSHTM CTU). A detailed statistical analysis plan (SAP) will be finalised prior to the unblinding of any data. The protocol (and statistical analysis plan) may be amended prior to the unblinding of any data as further testable hypotheses that can be accommodated easily into the design are identified. (Supplementary Appendix 4).
Trial conduct and governance
BHF PROTECT-TAVI is sponsored by the University of Oxford and run by the LSHTM CTU. The Trial Management Group (TMG) meets to coordinate the day-to-day management of the trial and ensure it is conducted within the guidelines of Good Clinical Practice, to protect participants and data integrity. The TMG is composed of investigators from the University of Oxford and the LSHTM CTU.
The trial is overseen by an independent trial steering committee (TSC) which meets as frequently as required, but no less frequently than once per year, for the duration of the trial. An independent data monitoring committee (DMC) reports to the TSC and examines the data accumulated during the progress of the BHF PROTECT-TAVI trial and ensures that the benefit/risk balance remains acceptable for participating patients. The frequency of meetings will depend upon trial progress with a minimum frequency of once per year.
An updated list of all the trial committees is available on the BHF PROTECT-TAVI website (https://www.lshtm.ac.uk/research/centres-projects-groups/bhfprotect-tavi).
Trial timelines and progress
BHF PROTECT-TAVI commenced set-up on 1 August 2020 and will report its results once active follow-up is complete, 12 months after the final patient is recruited.
BHF PROTECT-TAVI is designed with three milestones, which include two interim analyses for efficacy and futility. These were agreed with the funder and the DMC (Figure 2, Supplementary Appendix 5).
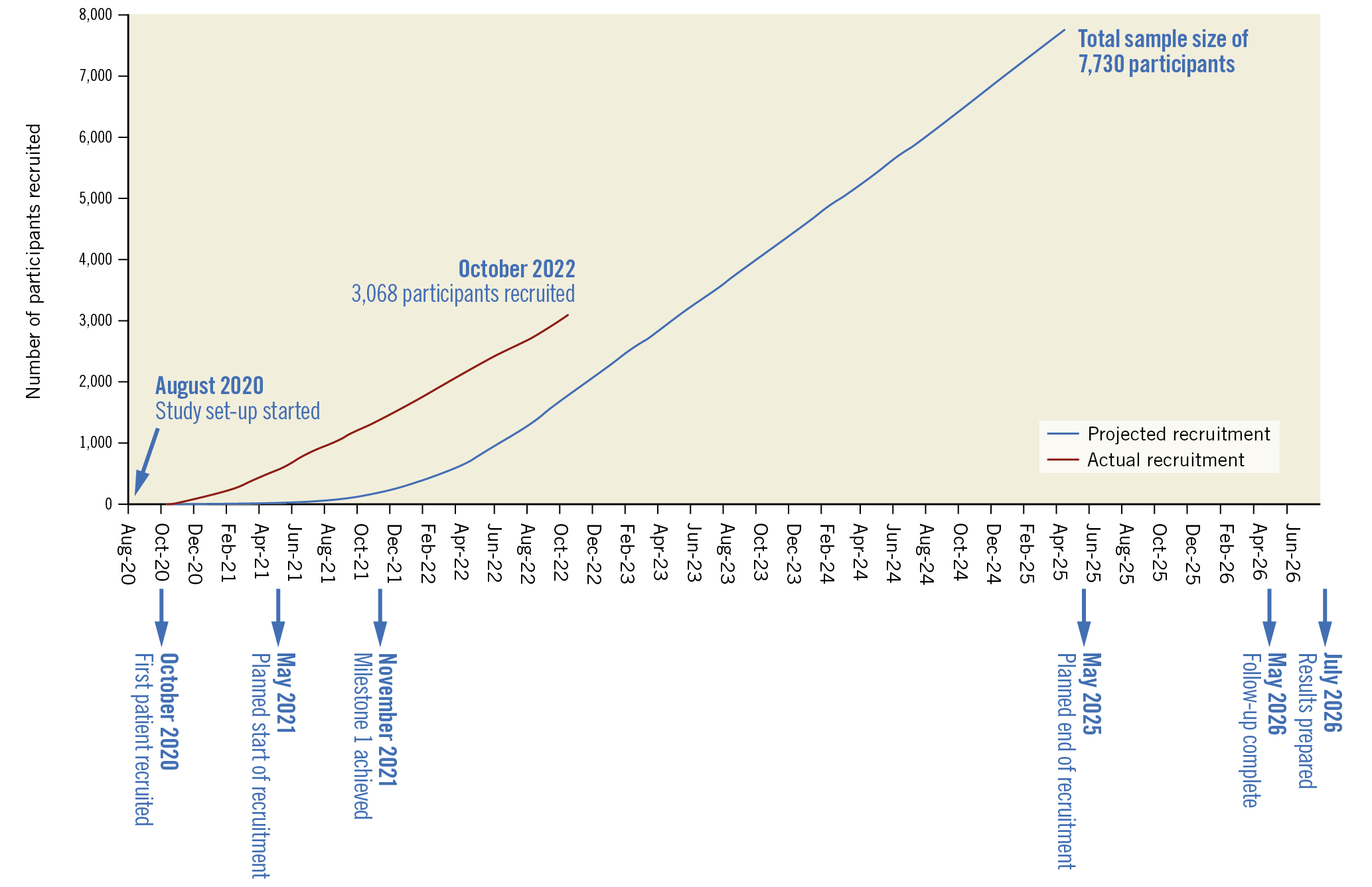
Figure 2. Trial progress and targets as of October 2022.
COVID-19 impact and mitigation
Despite the challenges posed by the COVID-19 pandemic, recruitment for the BHF PROTECT-TAVI trial opened on 29 October 2020, over 6 months ahead of target. Recruitment is planned to be completed by May 2025 with active follow-up concluding 12 months after the end of recruitment. As of October 2022, 3,068 participants have been recruited (Figure 2). To launch and conduct BHF PROTECT-TAVI during the COVID-19 pandemic, the trial team leveraged innovative techniques to minimise in-person contact, including remote site initiation visits, electronic site files and remote consent for participants.
Patient and public involvement
Meaningful patient and public involvement is fundamental to both the initial design and to the ongoing conduct of BHF PROTECT-TAVI. Research into managing stroke and developing new technologies to do so was prioritised by the Stroke Association Research Strategy (2019-2024) and the James Lind Alliance Priority Setting Partnership2122. Cerebral embolic protection specifically addresses these priorities.
Lay representatives on the TSC and the patient advisory group at the BHF were involved in outcome selection, follow-up schedule and trial design that was incorporated into protocol and patient documentation development. Two patient representatives are full members of the TSC.
Blinding
BHF PROTECT-TAVI is an open-label trial, and so, trial participants and hospital staff will be aware of the treatment allocation. The CEC will be blinded to trial treatment in adjudicating the primary outcome to limit bias.
Ethics and dissemination
Ethics
In the design of BHF PROTECT-TAVI, the trial team and co-investigators identified several issues.
In order to identify eligible patients, patient identifiable information will need to be screened prior to the patient providing consent. To address this, only members of the patient’s direct care team will be involved with patient screening.
There are risks associated with CEP: the increased exposure to radiation, infection at the insertion point and bleeding. To ensure that the risks of participating in the trial are clear to patients, they are fully discussed in the patient information sheet and presented in the accompanying video animation (https://www.explainmyprocedure.com/protecttavi/).
Ethical approval and oversight for BHF PROTECT-TAVI is provided by the Health Research Authority and Wales Research Ethics Committee 5 (REC 20/WA/0121; IRAS276396). There have been two substantial amendments to the protocol approved since recruitment started. The first clarified the adverse event reporting system, provided further information on how participants would be linked to routine data, added the animation explaining the trial to potential participants, and clarified when consenting patients should be randomised. The second added stroke severity as a secondary outcome.
Dissemination
Results will be disseminated through publications and conferences. BHF PROTECT-TAVI was prospectively registered on the ISRCTN (ISRCTN16665769). Results will be posted to ISRCTN.
Conclusions
CEP has the potential to reduce TAVI-associated stroke. This trial will assess the clinical efficacy and cost-effectiveness of a strategy of routine use of CEP in TAVI. This trial is currently recruiting, with 3,068 participants enrolled at the time of manuscript submission. The principal results are expected in 2025.
Acknowledgements
We particularly thank all participants and site staff for their support of the trial.
We thank the members of the trial steering committee, data monitoring committee and independent clincal event committee for their contributions, support and guidance of BHF PROTECT-TAVI. We especially thank all the lay representatives who have been involved in the development of the trial.
Funding
BHF PROTECT-TAVI is funded by the British Heart Foundation (BHF Clinical Study no. CS/20/1/34732). Funding for the CEP devices is provided by Boston Scientific, who are not involved in the coordination or conduct of the study.
Conflict of interest statement
A. Banning reports institutional grant support from Boston Scientific; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boston Scientific, Miracor, and Abbott. A. Baumbach reports consulting fees from PI Medical, Faraday, and SINOMED; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Medtronic, and MicroPort;and participation on a data safety monitoring board or advisory board for PI Medical and CERC. D.J. Blackman reports consulting fees from Medtronic, Abbott Vascular, and Edwards Lifesciences; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boston Scientific, Medtronic, Abbott Vascular, and Edwards Lifesciences; payment for expert testimony from Edwards Lifesciences; and support for attending meetings and/or travel from Edwards Lifesciences and Shockwave. D. Hildick-Smith reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boston Scientific. R.K. Kharbanda reports consulting fees from Boston Scientific and Edwards Lifesciences; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Boston Scientific and Edwards Lifesciences; and institutional grant support from Boston Scientific. M. Dodd reports institutional grant support from the British Heart Foundation. R. Evans reports institutional grant support from the British Heart Foundation. Z. Jamal reports institutional grant support from the British Heart Foundation. T. Clayton reports institutional grant support from the British Heart Foundation. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

