Abstract
Over the past decade, approximately 15,000 patients have been randomised in clinical trials of transcatheter aortic valve implantation (TAVI), and as many patients will be randomised in ongoing and future investigations aimed at broadening indications, comparing devices, simplifying the procedure, and optimising clinical outcomes. The purpose of this review is to summarise the rationale and design of upcoming studies in the field of TAVI.
Introduction
A decade after its introduction, with more than 250,000 procedures performed worldwide in more than 1,000 centres, and about 15,000 patients randomised in clinical trials, transcatheter aortic valve implantation (TAVI) is a respected treatment option for symptomatic patients with severe aortic stenosis1. However, judging from the large number of TAVI trials in the pipeline, one may argue that the journey of catheter-based treatments for aortic stenosis has just begun. The purpose of this review is to “look forward”, with a focus on the expectations and design of upcoming trials in the TAVI field. Ongoing and planned studies will be grouped according to four major themes: 1) expansion of clinical indications, 2) device comparisons, 3) procedural simplification, and 4) outcome optimisation.
Expanding TAVI indications
Current guidelines from the European Society of Cardiology and the American Heart Association/American College of Cardiology recommend TAVI for patients with severe symptomatic aortic stenosis and reasonable life expectancy who are inoperable (Class I) or high risk for surgery (Class IIa)2,3. A plethora of randomised studies whose goal is to expand these indications and fill important knowledge gaps is ongoing (Figure 1).
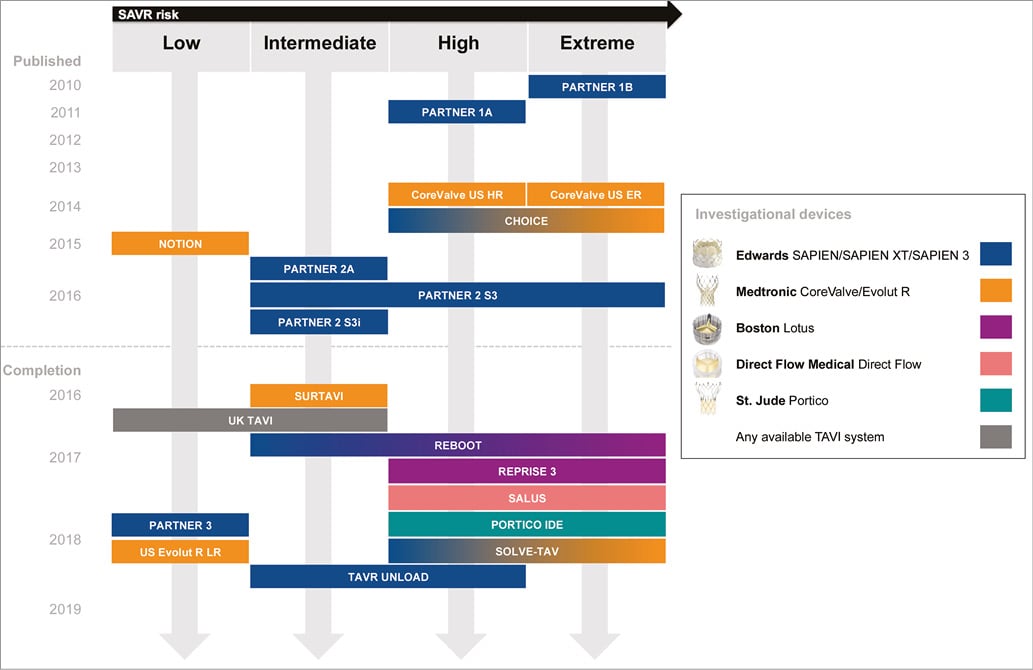
Figure 1. Timeline of published and selected ongoing studies of transcatheter aortic valve implantation across the spectrum of surgical risk. TAVI UNLOAD includes patients with moderate aortic stenosis and left ventricular ejection fraction >20% and <50%. For unpublished studies, the estimated primary completion date (final data collection date for primary outcome measure) is indicated as reported by the investigators in the clinicaltrials.gov or isrctn.com databases.
INTERMEDIATE SURGICAL RISK PATIENTS
The results of the PARTNER (Placement of Aortic Transcatheter Valves) 2A4 randomised trial and the SAPIEN 3i5 Propensity Score Analysis will inform future guidelines and probably contribute to broadening the scope of TAVI as an alternative or preferred treatment option for patients at intermediate surgical risk. SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation, NCT01586910) is a multicentre trial of the CoreValve® or Evolut R® transcatheter heart valve systems (Medtronic, Minneapolis, MN, USA) compared with surgical aortic valve replacement (SAVR) in intermediate-risk patients with a Heart Team predicted risk of 30-day mortality ≥3% and <8% (including calculation of the Society of Thoracic Surgeons predicted risk of surgical mortality [STS-PROM] score augmented by consideration of overall clinical status and comorbidities). The primary endpoint is all-cause mortality or disabling stroke at two years. Randomisation was completed in May 2016 with approximately 1,600 patients enrolled into the primary analysis cohort, and first data release is expected in the first quarter of 2017.
LOW SURGICAL RISK PATIENTS
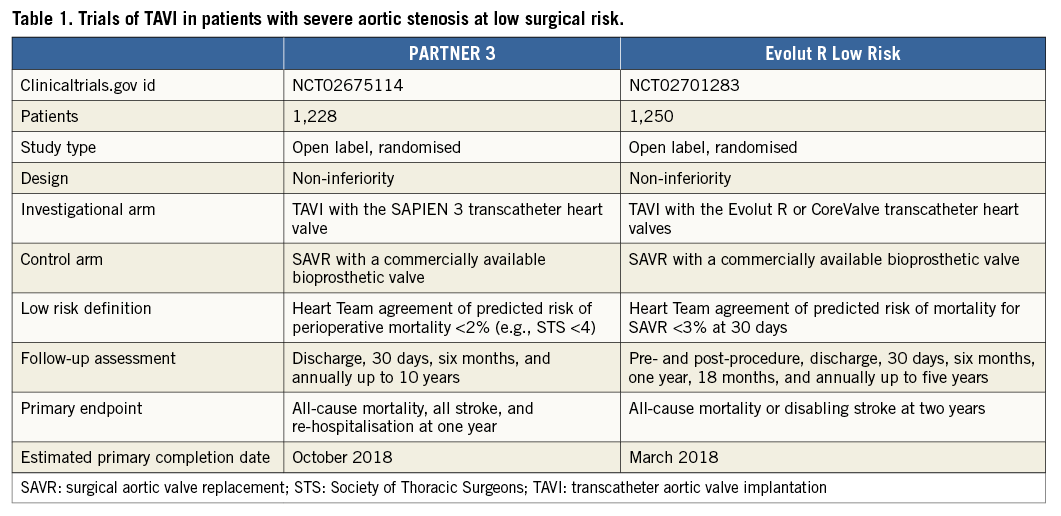
With a focus on intermediate but especially low-risk patients, UK-TAVI (ISRCTN57819173) will include 808 “all-comer” patients, randomised to TAVI at 30 sites in the United Kingdom with any commercially available TAVI device, or SAVR. The primary endpoint is all-cause death at one year with additional cost-effectiveness analyses. Two other non-inferiority trials using balloon-expandable (PARTNER 3, NCT02675114) and self-expanding prostheses (Evolut R Low Risk, NCT02701283) have recently initiated the recruitment of patients with Heart Team agreement of predicted perioperative mortality <2% and <3%, respectively (Table 1). PARTNER 3 randomises transfemoral TAVI with the SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA) vs. SAVR with a bioprosthetic valve with a primary composite endpoint at one year including all-cause mortality, all strokes, and re-hospitalisation. The Evolut R Low Risk trial randomises TAVI with the Evolut R and CoreValve prostheses compared vs. SAVR with a bioprosthetic valve with a primary composite endpoint at two years including all-cause mortality or disabling stroke.
MODERATE AORTIC STENOSIS PATIENTS WITH HEART FAILURE
Left ventricular dysfunction is a frequent coexisting condition in patients with aortic stenosis. In this setting, the management of patients with moderate aortic stenosis and clinical heart failure is frequently debated. In TAVR UNLOAD (Transcatheter Aortic Valve Replacement to UNload the Left Ventricle in Patients with ADvanced Heart Failure, NCT02661451), 600 patients with moderate aortic stenosis, symptomatic heart failure despite optimal medical therapy, and depressed left ventricular ejection fraction (>20% and <50%) will be randomised to TAVI with the SAPIEN 3 valve or optimal medical therapy alone. The two strategies will be investigated with respect to the hierarchical occurrence of all-cause mortality, disabling stroke, hospitalisations related to heart failure, or change in the Kansas City Cardiomyopathy Questionnaire at one year.
Comparing TAVI options
Three active-controlled randomised trials of TAVI devices are underway to generate efficacy data to support Food and Drug Administration clearance of transcatheter heart valves already approved for commercial use in Europe (Figure 1). All of these studies will target high- and extreme-risk patients with severe aortic stenosis: 1) PORTICO IDE (N=908, NCT02000115) is comparing the Portico™ (St. Jude Medical, St. Paul, MN, USA) valve and either SAPIEN or CoreValve systems; 2) SALUS (TranScatheter Aortic Valve RepLacement System Pivotal Trial The Safety and Effectiveness of the Direct Flow Medical Transcatheter Aortic Valve System) (N=649, NCT02163850) is comparing the Direct Flow Medical® (Direct Flow Medical, Santa Rosa, CA, USA) with the SAPIEN or CoreValve systems; 3) REPRISE III (Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus™ Valve System) (N=1,032, NCT02202434) is a comparison of the Lotus™ (Boston Scientific, Marlborough, MA, USA) and CoreValve systems. A small investigator-initiated trial named REBOOT (REpositionable Versus BallOOn-expandable Prosthesis for Trans-catheter Aortic Valve Implantation) (N=240, NCT02668484) is comparing the Lotus and SAPIEN 3 valves with respect to the incidence of new pacemaker implantation at 30 days. An industry-driven randomised trial of the ACURATE neo™ valve (Symetis, Ecublens, Switzerland) vs. other TAVI systems is also planned.
Other new TAVI systems are in earlier phases of clinical testing as part of ongoing single-arm studies. These include the Centera valve (Edwards Lifesciences), whose safety and performance are the object of CENTERA-EU (N=200, NCT02458560), and the JenaValve Pericardial TAVR System (JenaValve, Munich, Germany), which will be tested in two feasibility trials of high-risk patients with aortic stenosis (N=30, NCT02732691) or aortic regurgitation (N=30, NCT02732704) in Europe and the USA.
Simplifying the TAVI procedure
Multiple small trials are building on the concept of a “minimalist” approach by streamlining specific steps of the TAVI procedure. These include: 1) SOLVE-TAV (SecOnd-generation seLf-expandable Versus Balloon-expandable Valves and gEneral Versus Local Anesthesia in TAVI) (N=444, NCT02737150), a factorial study of Evolut R versus SAPIEN 3 and local anaesthesia with conscious sedation versus general anaesthesia with respect to periprocedural events; 2) DIRECT (The preDIlatation in tRanscathEter aortiC Valve implanTation Trial) (N=170, NCT02448927), a trial of direct versus non-direct TAVI (i.e., with or without balloon aortic valvuloplasty before TAVI) targeting device success; and 3) EASY TAVI (Direct Left Ventricular Rapid Pacing Via the Valve Delivery Guide-wire in TAVI) (N=400, NCT02781896), a trial of left ventricular rapid pacing using the valve delivery guidewire compared with rapid pacing by a standard right ventricular pacing catheter, targeting a reduction in procedure duration.
Optimising TAVI outcomes
CONCOMITANT CORONARY ARTERY DISEASE
Whether concurrent coronary artery disease found in the setting of TAVI workup must be treated before the procedure is a dilemma. The issue will be assessed by ACTIVATION (percutAneous Coronary inTerventIon prior to transcatheter aortic VAlve implantaTION) (ISRCTN75836930), a trial of 310 patients randomised to treatment of significant coronary artery disease by percutaneous coronary intervention (PCI) or no PCI. The trial tests the hypothesis that the strategy of performing pre-TAVI PCI is comparable to not treating such coronary stenoses, with respect to a composite primary outcome of one-year mortality and re-hospitalisation.
CONTRAST-INDUCED NEPHROPATHY
Contrast-induced acute kidney injury jeopardises the outcomes of patients undergoing TAVI6. REDUCE AKI (Reducing Acute Kidney Injury in TAVI Patients) (N=220, NCT01866800) is evaluating whether forced diuresis with matched hydration using the RenalGuard® system (RenalGuard Solutions, Milford, MA, USA) is effective in reducing the risk of contrast-induced acute kidney injury compared with conventional treatment.
CEREBROVASCULAR EMBOLIC PROTECTION
Two trials are ongoing to evaluate the impact of embolic protection with the Sentinel™ (Claret Medical, Santa Rosa, CA, USA) and TriGuard™ (Keystone Heart, Caesarea, Israel) systems. In the SENTINEL IDE trial, 356 patients have been randomised 1:1:1 to embolic protection with the Sentinel device (safety and test cohorts) or no embolic protection, with the aim of evaluating 1) the composite of death, stroke or acute kidney injury in the safety cohort with respect to a pre-specified objective performance goal, and 2) the reduction in median total new lesion volume between the test and control cohorts, assessed using diffusion-weighted magnetic resonance imaging studies at two to seven days after the TAVI procedure. Similarly, in REFLECT (A Randomized Evaluation of the TriGuard Embolic Deflection Device to Reduce the Impact of Cerebral Embolic Lesions After Transcatheter Aortic Valve Implantation) (N=285, NCT02536196), the TriGuard device will be compared with unprotected TAVI with respect to a hierarchical composite endpoint including major adverse cardiac or cerebrovascular events, neurocognitive function, and the total volume of cerebral ischaemic lesions detected by diffusion-weighted magnetic resonance imaging.
ANTITHROMBOTIC THERAPY
The multiple strategies currently being investigated reflect the extreme uncertainty surrounding the topic of antithrombotic management in the TAVI scenario. The ARTE (Aspirin Versus Aspirin + ClopidogRel as Antithrombotic Treatment Following Transcatheter Aortic Valve Implantation With the Edwards SAPIEN XT Valve) trial (NCT01559298) is evaluating the efficacy of aspirin monotherapy (80 mg/day for six months) versus the combination of aspirin (80 mg/day for six months) and clopidogrel (75 mg/day for three months) in 200 patients with no indication for oral anticoagulation with respect to the combination of death, myocardial infarction, stroke or transient ischaemic attack and major bleeding at one year. POPULAR TAVI (Antiplatelet Therapy for Patients Undergoing Transcatheter Aortic Valve Implantation) (N=1,010, NCT02247128) aims to demonstrate similar efficacy and better safety for a clopidogrel alone regimen (75 mg/day for three months) versus either the combination of clopidogrel 75 mg/day for three months plus aspirin 100 mg/day for one year in patients with no atrial fibrillation or oral anticoagulation in patients with atrial fibrillation. AUREA (Dual Antiplatelet Therapy Versus Oral Anticoagulation for a Short Time to Prevent Cerebral Embolism After TAVI) (N=124, NCT01642134) is comparing the fixed combination of aspirin 80 mg/day and clopidogrel 75 mg/day versus oral anticoagulation with acenocoumarol in patients with no indication for oral anticoagulation, with respect to the occurrence of thromboembolic cerebrovascular events by magnetic resonance imaging at three months. AVATAR (Anticoagulation Alone Versus Anticoagulation and Aspirin Following Transcatheter Aortic Valve Interventions) (N=170, NCT02735902) aims to demonstrate that a single anticoagulant therapy is superior to a combination of anticoagulant and antiplatelet therapy with respect to a net clinical benefit endpoint at one year.
Two more studies are testing non-vitamin K-antagonist oral anticoagulants for the long-term prevention of thrombotic valvular events. GALILEO (Global Study Comparing a rivAroxaban-based Antithrombotic Strategy to an antipLatelet-based Strategy After Transcatheter aortIc vaLve rEplacement to Optimize Clinical Outcomes) (NCT02556203) is a multicentre study conducted at 100 international sites to compare oral anticoagulation with rivaroxaban (10 mg/day long term, coupled with aspirin in the first three months) versus aspirin (75-100 mg/day long term, coupled with clopidogrel in the first three months) in 1,520 patients with no prior indication for oral anticoagulation. The idea behind the design of GALILEO is the de-escalation of antithrombotic therapy by dropping one antiplatelet agent at three months (aspirin in the investigational group, clopidogrel in the control group), and continuing with a head-to-head comparison of rivaroxaban and aspirin from three months to the end of the study period. The primary efficacy endpoint is the composite of all-cause death, stroke, systemic embolism, myocardial infarction, pulmonary embolism, deep venous thrombosis and symptomatic valve thrombosis. The primary safety endpoint is the composite of life-threatening, disabling and major bleeding events. Different from GALILEO, ATLANTIS (Anti-Thrombotic Strategy to Lower All Cardiovascular and Neurologic Ischemic and Hemorrhagic Events After Trans-Aortic Valve Implantation for Aortic Stenosis) (N=1,509, NCT02664649) embraces a broader spectrum of TAVI patients (i.e., those with and without atrial fibrillation) to compare apixaban (5 mg/bid or 2.5 mg/bid in selected clinical scenarios) versus standard-of-care (vitamin K antagonists in patients with indications for oral anticoagulation, or single/double antiplatelet therapy in patients with no indication for oral anticoagulation). The primary endpoint of ATLANTIS is the composite of death, myocardial infarction, systemic emboli, intracardiac or bioprosthetic thrombus, deep venous thrombosis, pulmonary embolism or major bleeding at one year.
VALVE LEAFLET INTEGRITY, THICKENING AND THROMBOSIS
The incidence and clinical implications of valve leaflet thickening and thrombosis are the object of multiple ongoing 4D computed tomography imaging studies targeting the cumulative enrolment of more than 2,000 patients. RESOLVE (Assessment of TRanscathetEr and Surgical Aortic BiOprosthetic Valve Thrombosis and Its TrEatment With Anticoagulation) (NCT02318342) is designed to evaluate prospectively the structural and functional integrity of transcatheter or surgical bioprosthetic aortic valves with multimodality imaging (Phase 1). The study further aims to confirm resolution of any early bioprosthetic valve thrombotic changes with vitamin K antagonists (Phase 2). Similarly, SAVORY (Subclinical Aortic Valve biOprosthesis thRombosis Assessed With 4D Computed tomographY) (NCT02426307) is an ongoing registry designed to assess the frequency of subclinical abnormal leaflet motion and morphology in patients treated with transcatheter or surgical aortic valve bioprosthesis, and to monitor the natural evolution of this phenomenon in terms of clinical events and their relation to medical treatment. Further 4D computed tomography randomised (TAVI versus surgery) substudies have been included as part of the PARTNER 3 (N=400), Evolut R Low Risk (N=400) and PORTICO IDE (N=200) trials.
Conclusions
Upcoming TAVI studies are expected to address knowledge gaps while expanding current indications and refining clinical outcomes both acutely and during long-term follow-up. In parallel with approval studies of new technologies and iterations of current TAVI devices and long-term durability data from published studies, other unknown areas will probably be the objective of future trial design, including patients with low flow-low gradient aortic stenosis, severe asymptomatic aortic stenosis, and isolated aortic regurgitation.
Conflict of interest statement
D. Capodanno has no conflicts of interest to declare. M. Leon is a non-paid consultant for Edwards Lifesciences on the Executive Committee of the PARTNER trials.

