
Abstract
Aortic stenosis and mitral regurgitation are increasingly treated by percutaneous interventions including transcatheter aortic valve implantation (TAVI) and several mitral valve repair techniques, changing the landscape of valvular therapies in which surgery was predominant. Several randomised studies on TAVI have led to the use of this procedure in patients at intermediate or higher operative risk and have set strong foundations for future trials aiming to expand indications or to overcome several residual issues with TAVI. On the other hand, randomised evidence for percutaneous mitral valve repair (PMVR) techniques is still limited, supporting restricted indications to patients with high surgical risk when medical therapy fails. However, in the mitral field, several ongoing trials comparing PMVR with medical therapy or surgery will help to define optimal mitral regurgitation management in this era of evolving catheter-based treatment options. The present review will summarise randomised trials comparing TAVI or PMVR with medical therapy or surgery across the risk spectrum which have set the basis for guideline recommendations and for clinical use of transcatheter interventions. Characteristics, results, implications, unresolved issues and cost-effectiveness analysis of those trials, grouped according to the surgical risk of enrolled patients, will be appraised.
Abbreviations
AF: atrial fibrillation
AR: aortic regurgitation
AS: aortic stenosis
ICERs: incremental cost-effectiveness ratios
LVEF: left ventricular ejection fraction
MR: mitral regurgitation
PMVR: percutaneous mitral valve repair
PPI: permanent pacemaker implantation
PROM: predicted risk of mortality
PVL: paravalvular leak
SAVR: surgical aortic valve replacement
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
Introduction
Aortic stenosis (AS) and mitral regurgitation (MR) are the most prevalent heart valve pathologies, negatively impacting on quality of life and survival1,2. These two insidious valvular diseases are increasingly treated by percutaneous interventions including transcatheter aortic valve implantation (TAVI) and several mitral valve repair/implantation techniques, changing the landscape of valvular therapies in which surgery was predominant. Clearly, global TAVI adoption exceeds that of percutaneous mitral valve repair (PMVR) techniques, which may attest to the relatively simple tubular anatomy of the aortic valve and complex anatomic confluence of the mitral valve3,4. Moreover, multiple randomised trials on TAVI have supported expanding indications to include patients at progressively lower surgical risk. Conversely, the restriction of randomised trials on PMVR to one trial at least partially explains the limited indications for use. This review will summarise all randomised trials comparing TAVI or PMVR with medical therapy or surgery across the risk spectrum, which have set the basis for guideline recommendations and for clinical use of transcatheter interventions. Characteristics, results, implications, unresolved issues and cost-effectiveness analysis of those trials, grouped according to the surgical risk of enrolled patients, will be appraised.
Trials of TAVI versus medical therapy in inoperable patients
The landmark Placement of Aortic Transcatheter Valves (PARTNER) 1B randomised trial has demonstrated the superiority of TAVI compared with standard therapy in patients (n=358) with symptomatic severe AS who were not considered suitable for surgical aortic valve replacement (SAVR)5,6. At one year, transfemoral TAVI of a balloon-expandable bioprosthetic valve (Edwards SAPIEN; Edwards Lifesciences, Irvine, CA, USA) was associated with reduced rates of all-cause and cardiovascular death compared with standard therapy, including balloon aortic valvuloplasty performed in 83.8% of patients (30.7% and 20.5% vs. 50.7% and 44.6%, respectively, p<0.001). A sustained benefit of TAVI, as measured by mortality, re-hospitalisation, and functional status, was shown at five years6. Five-year mortality was 71.8% in TAVR and 93.6% in the standard treatment group, with a number needed to treat of five patients to save one life at one year follow-up. Five-year cardiovascular-related mortality was 57.5% in the TAVR group and 85.9% in the standard treatment group, suggesting that non-cardiovascular comorbidities were an important cause of death. This striking benefit in long-term mortality with TAVI in inoperable patients was achieved without significant increase in 30-day all-cause death (5.0% in TAVI vs. 2.8% in standard therapy groups, p=0.41). More neurologic events, major vascular complications, and major bleeding events occurred in the TAVI group than in the standard therapy group. Of note, beyond early procedural risk of stroke, there was no persistent risk over five years.
Trials on TAVI versus SAVR in high surgical risk patients
Two large randomised trials, the PARTNER 1A and U.S. CoreValve, have assessed the safety and effectiveness of TAVI with a balloon-expandable (SAPIEN) and a self-expanding (CoreValve®; Medtronic, Minneapolis, MN, USA) prosthesis, respectively, as compared with SAVR in patients with severe AS who were at high surgical risk7-10. Both trials assessed the non-inferiority of TAVI vs. SAVR for the primary endpoint of one-year all-cause death. The definition of high risk and the clinical outcomes of those two trials are described below.
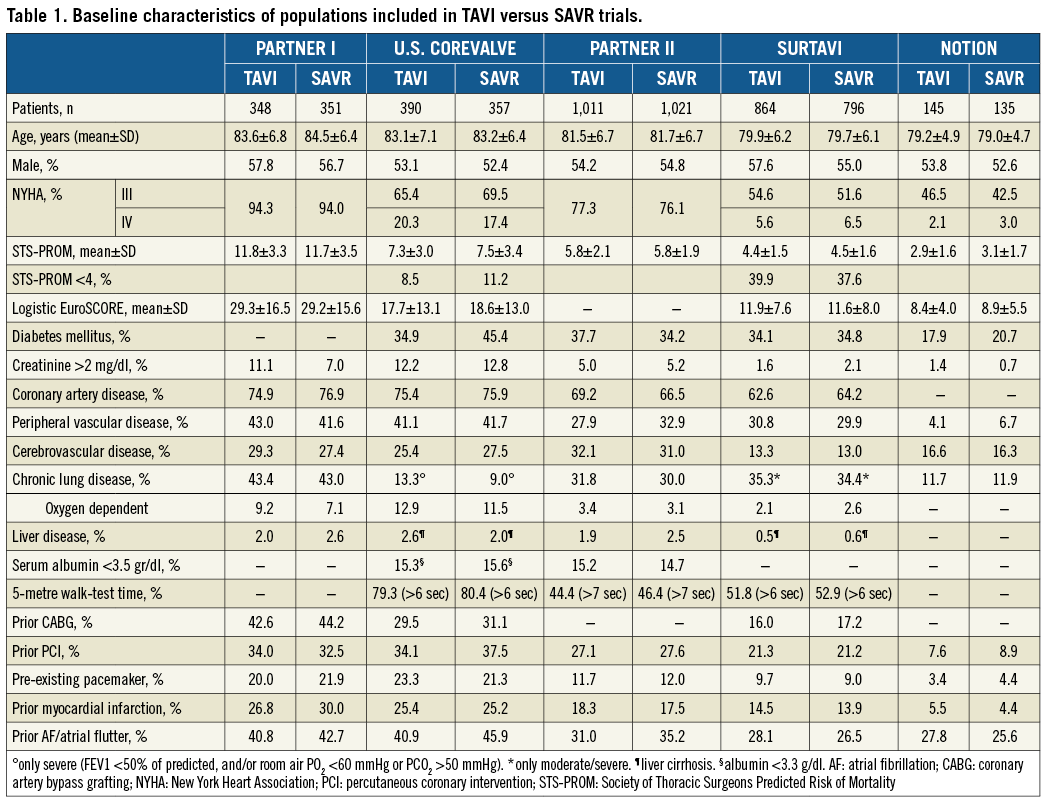
DEFINITION OF “HIGH” SURGICAL RISK IN TRIALS
In the PARTNER 1A trial patients deemed at high operative risk were required to have coexisting conditions such that the predicted risk of operative mortality was ≥15% and/or they had a Society of Thoracic Surgeons (STS) score of ≥10. In the U.S. CoreValve trial, patients were considered to be at increased surgical risk if the estimated 30-day risk of death was ≥15% and the risk of death or irreversible complications was less than 50%, based on STS-predicted risk of mortality (PROM) estimate and additional factors. As a result, the calculated STS PROM in the U.S. CoreValve trial was lower than in the PARTNER 1A trial (Table 1), suggesting large heterogeneity in risk assessment even in patients considered in general at “high” risk, especially when combining STS score with other coexisting factors. Indeed, in the PARTNER 1A trial the mean STS score was 11.8%, suggesting a higher operative risk (Table 1). Differently, in the U.S. CoreValve trial, the overall mean STS score was 7.4%, with most patients (74% of the overall population) having an STS score ranging from 4 to 10%, and 10% of patients with an STS score <4%.
CLINICAL OUTCOMES OF TAVI VERSUS SAVR IN “HIGH” RISK PATIENTS
The 30-day and one-year outcomes are reported in Table 2 and Table 3, respectively. Thirty-day all-cause death tended to be lower in the TAVI than in the SAVR group in the PARTNER 1A trial (5.2% vs. 8.0%, p=0.15), despite observed better-than-predicted surgical outcomes. In the U.S. CoreValve trial, all-cause mortality at 30 days was similar between TAVR and SAVR (3.3% vs. 4.5%, p=0.43, respectively). This difference in 30-day mortality trends in the two trials is probably due to the lower-risk population enrolled in the U.S. CoreValve trial, leading to a lower than expected surgical mortality. With respect to one-year all-cause death (the primary endpoint), TAVI was non-inferior to SAVR in both trials. Indeed, in PARTNER 1A, rates of all-cause death were similar between TAVI and SAVR at one year (24.2% vs. 26.8%, p=0.44) and five years (67.8% vs. 62.4%, p=0.76). The slightly higher mortality rate after 30 days in the TAVI group of the PARTNER 1A trial was mostly driven by non-cardiovascular reasons. In the CoreValve U.S. trial, one-year all-cause mortality was significantly lower in TAVI vs. SAVR (14.2% vs. 19.1%, p<0.0001 for non-inferiority and p=0.04 for TAVI superiority) and remained lower at three years (32.9% vs. 39.1%, p=0.07). Of note, the lower-risk population enrolled in the U.S. CoreValve trials is also reflected in the lower one-year mortality rates observed in this trial compared with PARTNER 1A. In summary, it seems that, in cohorts defined as “high” risk, TAVI is associated with mortality benefit, which emerged earlier, as reduced periprocedural hazard, in those at relatively higher risk. However, the case for superiority of TAVI in terms of one-year mortality reported in the U.S. CoreValve trial is less robust. Indeed, superiority would not have been established if the one-sided alpha level of 0.025 had been used in the trial, in which a one-sided alpha level of 0.05 was applied.
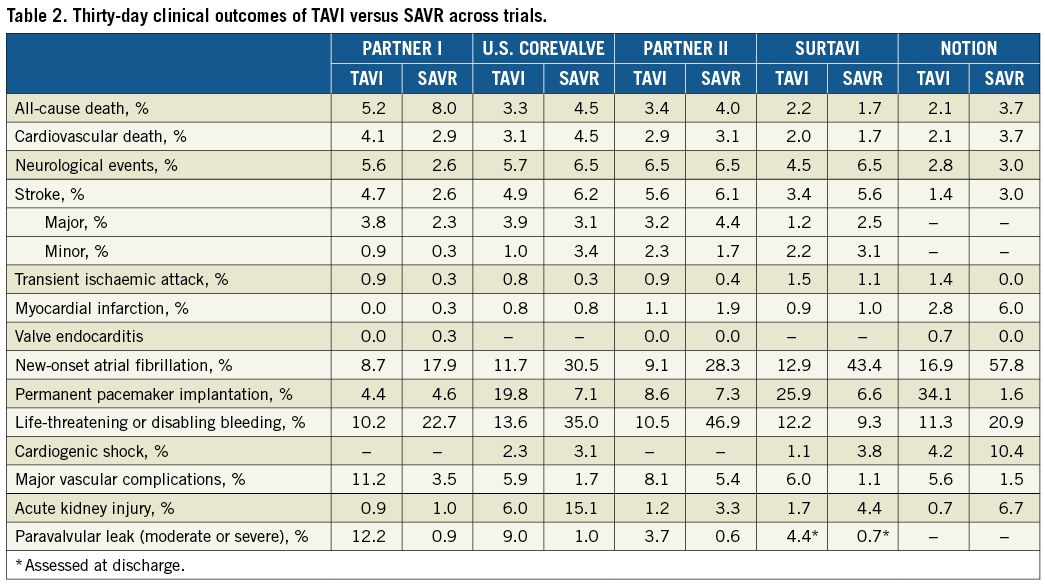
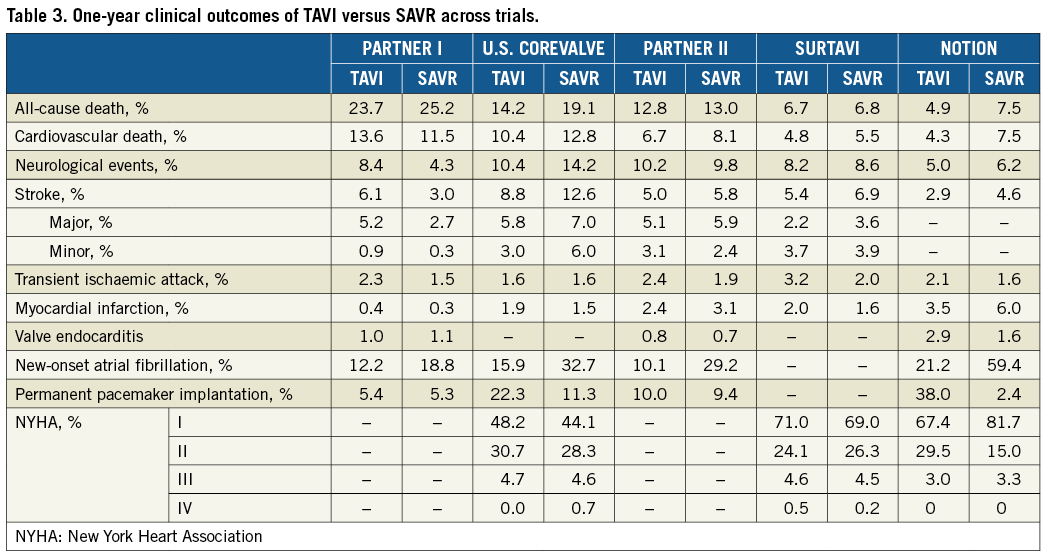
While in the PARTNER 1A trial 30-day and one-year rates of all strokes and transient ischaemic attacks and major stroke were higher in the transcatheter group than in the surgical group, no significant differences between TAVI and SAVR were observed for these neurologic outcomes in the U.S. CoreValve trial.
In both the PARTNER 1A and U.S. CoreValve trials, the TAVI group had significantly higher 30-day rates of major vascular complications than did the surgical group but had lower rates of major bleeding and new-onset atrial fibrillation (AF) (Table 2). New permanent pacemaker implantation (PPI) did not differ significantly between TAVI and SAVR in the PARTNER 1A trial at both one (6.4% vs. 5.3%) and five years (9.7% vs. 9.1%). Conversely, more pacemakers were implanted in TAVI than SAVR patients at one year (22.3% vs. 11.3%) and three years (28.0% vs. 14.5%) in the U.S. CoreValve trial. Finally, moderate-severe paravalvular leak (PVL) at one year was more frequent in the TAVI than in the SAVR group (6.8% vs. 1.9% in PARTNER 1A, and 6.1% vs. 0.5% in the U.S. CoreValve trial).
Trials on TAVI versus SAVR in intermediate surgical risk patients
Two randomised trials, PARTNER 2A and SURTAVI11,12, have assessed the safety and effectiveness of TAVI as compared with SAVR in patients with symptomatic severe AS considered at intermediate surgical risk.
Both trials assessed the non-inferiority of TAVI vs. SAVR for the primary endpoint of all-cause death or disabling stroke at 24 months. The TAVI procedure was performed with the second-generation balloon-expandable valve system (SAPIEN XT; Edwards Lifesciences) in the PARTNER 2A trial and with a self-expanding bioprosthesis (first-generation CoreValve in 84% and the second-generation Evolut™ R [Medtronic] in 16%) in the SURTAVI trial. Intermediate risk definition and clinical outcomes of those trials are described below.
DEFINITION OF “INTERMEDIATE” RISK IN TRIALS
The intermediate risk for surgery in PARTNER 2A was defined as an STS score ≥4% and <8%, or <4% in the presence of coexisting comorbidities not represented in the STS risk score algorithm, as assessed by the Heart Team.
In SURTAVI, intermediate risk was defined as a predicted risk of operative mortality ≥3% and <15% at 30 days, based on STS score augmented by consideration of the overall clinical status and comorbidities unmeasured by the STS risk calculation (i.e., age ≥75 years, frailty indices, major organ disease, etc.).
The overall risk in the SURTAVI trial appeared lower than in PARTNER 2A (Table 1). Of note, in the SURTAVI trial most patients (66.4%) had an STS score <5% and a relevant proportion had a score <4% (38.8%).
CLINICAL OUTCOMES OF TAVI VERSUS SAVR IN “INTERMEDIATE” SURGICAL RISK PATIENTS
The TAVI procedure was non-inferior to SAVR with respect to the primary composite endpoint of all-cause death or disabling stroke at two years in the PARTNER 2A and SURTAVI trials (Figure 1A).
All-cause death rates were not significantly different at 30 days, one year and two years in both trials (Table 2-Table 4). Of note, a very low 30-day surgical mortality was observed in SURTAVI (1.7% compared with 4.0% observed in the PARTNER 2A trial), achieving the lowest observed-to-expected 30-day surgical mortality ratio (0.38 vs. 0.71 in the PARTNER 2A trial). The excellent surgical results observed in the SURTAVI trial, attesting to the best practice of the surgical teams involved, underscore the importance of the lack of difference in mortality between TAVI and SAVR (1.7% vs. 2.2% at 30 days), and probably reflect the lower-risk population enrolled in the SURTAVI trial. The fact that in the SURTAVI trial patients were at a lower risk is also supported by the lower absolute rates of two-year mortality observed in SURTAVI (11.4% in TAVI vs. 11.6% in SAVR) than in the PARTNER 2A trial (16.7% in TAVI vs. 18.0% in SAVR).
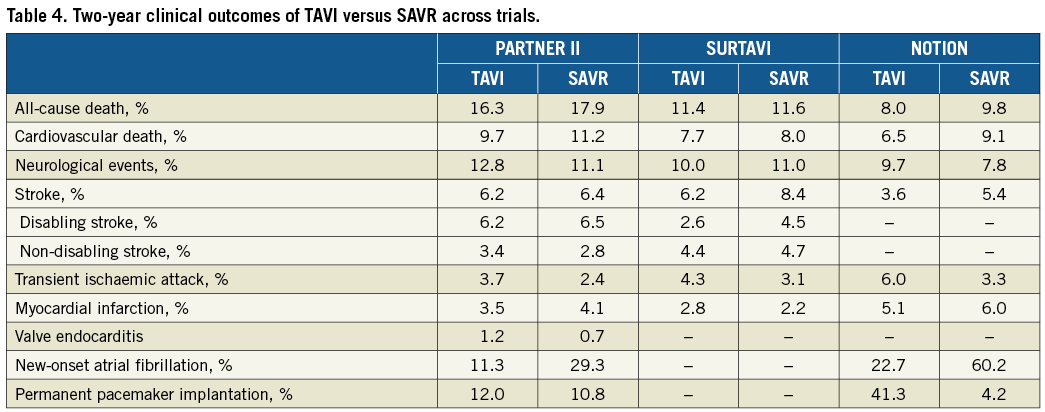
Similar rates of disabling stroke were observed between TAVI and SAVR in the overall population of the PARTNER 2A trial, while numerically lower events occurred with TAVI than with SAVR at any time point in the SURTAVI trial, with the difference reaching the limit of statistical significance at two years (Table 2-Table 4, Figure 1B).
With respect to endpoints other than mortality and stroke, consistent with findings in high-risk cohorts, among patients considered at “intermediate” surgical risk the TAVI procedure was associated with fewer life-threatening or major bleeding events, less acute kidney injury and new-onset AF, as well as a more rapid recovery and a shorter in-hospital stay compared with SAVR. Conversely, TAVI was associated with more major vascular complications, PVL and new PPI.
In summary, based on the PARTNER 2A and SURTAVI trials, TAVI was non-inferior to SAVR with respect to mortality or stroke with trends towards reduced disabling stroke in patients at intermediate operative risk. However, comparison of these outcomes might significantly favour TAVI vs. SAVR if a new-generation valve were to be used. This hypothesis was generated in a propensity score analysis of intermediate-risk patients13, in which TAVI with SAPIEN 3 (Edwards Lifesciences), a third-generation balloon-expandable valve (from the PARTNER SAPIEN 3 registry) was superior to SAVR (from the PARTNER 2A trial) for the composite of all-cause death, any stroke, and moderate-severe aortic regurgitation (AR), with a significant pooled weighted proportion difference (–9.2%, 95% confidence interval [CI]:–13 to –5.4). Furthermore, TAVI with the SAPIEN 3 resulted in better outcome than TAVI with the SAPIEN XT from the PARTNER 2A trial14. All-cause death was 1.1% at 30 days and 7.4% at one year (vs. 3.9% and 12.3% in the PARTNER 2A TAVI cohort). At 30 days, rates of disabling stroke (1.0% vs. 3.2%), myocardial infarction (0.3% vs. 1.2%), major vascular complications (6.1% vs. 7.9%), life-threatening or disabling bleeding (4.6% vs. 10.4%) and new AF (5.0% vs. 9.1%) were all numerically lower in the SAPIEN 3 registry than in the TAVI group of the PARTNER 2A trial, in which a SAPIEN XT valve was used.
TAVI versus SAVR in low-risk patients
The proven benefits of TAVI in higher-risk patients are leading to expansion of its indications to low-risk populations who account for 80% of AS patients undergoing surgery15. However, limited knowledge is currently available on the safety and effectiveness of TAVI in patients at low surgical risk who could be defined by an STS score of less than 4% without additional coexisting comorbidities or frailty conditions. Preliminary comparative data of TAVI vs. surgery in patients at low risk can be derived from the Nordic Aortic Valve Intervention (NOTION) trial and from registries based on propensity score-matched analysis16-18. NOTION was a superiority all-comers trial assessing one-year rates of the primary composite endpoint of all-cause death, stroke or myocardial infarction in patients ≥70 years old with severe AS and without coronary artery disease who were randomised to TAVI with a CoreValve self-expanding bioprosthesis versus SAVR, regardless of their predicted risk of death after surgery16,17. In the NOTION trial, patients (n=280) had a mean STS score of 3.0±1.7%, and 82% had an STS <4% (Table 1). Also, comorbidities (e.g., diabetes, lung disease, renal insufficiency, cerebrovascular disease, etc.) were markedly less prevalent in NOTION compared with the other trials (Table 1). Rates of the primary endpoint were similar between TAVI, performed by transfemoral approach in 96.5%, and SAVR at one year (13.1% vs. 16.3%; –3.2% absolute difference; p=0.43 for superiority) and two years (15.8% vs. 18.8%, p=0.43). Moreover, rates of any death and all stroke were numerically lower in TAVI vs. SAVR at one and two years (Table 3, Table 4). Of note, new PPI occurred at a rate as high as 34%. Although the overall results of the NOTION trial were promising, the trial was largely underpowered and represented a highly selected group of patients (only 18% of the screened patients). The increasing experience with TAVI, the better outcomes and low complication rates have prompted FDA approval of two non-inferiority trials using balloon-expandable (PARTNER 3, NCT02675114) and self-expanding prostheses (Evolut R Low Risk, NCT02701283) on low-risk patients with predicted perioperative mortality <2% and <3%, respectively19. The PARTNER 3 trial randomised transfemoral TAVI with the SAPIEN 3 valve vs. SAVR with a bioprosthetic valve with a primary composite endpoint at one year including all-cause mortality, all strokes, and re-hospitalisation. The Evolut R Low Risk trial randomises TAVI with the Evolut R and CoreValve prostheses vs. SAVR with a bioprosthetic valve with a primary composite endpoint at two years including all-cause mortality or disabling stroke. Results of both trials are expected in early 2019. NOTION-2 (NCT02825134) is another ongoing trial of TAVI vs. SAVR in low-risk patients <75 years old. Moreover, two ongoing trials are assessing novel TAVI indications among low-risk patients. The Early TAVR randomised trial (NCT03042104) compares TAVI with the SAPIEN 3 valve vs. clinical surveillance in asymptomatic patients with severe AS. The ongoing TAVR UNLOAD trial (NCT02661451) compares TAVI with the SAPIEN 3 valve versus optimal heart failure therapy only in patients with symptomatic heart failure, left ventricular ejection fraction (LVEF) <50%, but >20%, and moderate AS20.
TAVI versus SAVR according to access route
Clinical outcomes of TAVI vs. SAVR stratified by route of access were reported in the PARTNER trials7,11. In the as-treated analysis of the PARTNER 1A trial7, 30-day all-cause death tended to be significantly lower in the TAVI than in the SAVR group (3.7% vs. 8.2%, p=0.046) among the transfemoral placement cohort, while no significant differences were observed in the transapical placement cohort (8.7% vs. 7.6%, p=0.79). There were no significant differences in the one-year rates of death between the transfemoral placement cohort and SAVR (powered comparison, absolute difference –4.2% with TAVI, p=0.002 for non-inferiority), or between the transapical placement cohort and the surgical group (unpowered comparison, absolute difference +3.8% with TAVI). In the PARTNER 1A trial, rates of major stroke were similar with TAVI vs. SAVR whether the access was transfemoral or transapical, with the highest absolute rates observed among the transapical TAVI group (7.0% vs. 2.5% in the transfemoral TAVI group at 30 days).
In the PARTNER 2A trial11, TAVI yielded lower rates of the primary composite endpoint of all-cause death or disabling stroke at two years among the transfemoral access cohort (Figure 1A), while outcomes were similar in the two groups in the transthoracic access cohort (transapical or transaortic). Splitting this composite endpoint, in the PARTNER 2A transfemoral cohort, TAVI vs. SAVR was associated with a trend towards lower two-year mortality (13.7% vs. 16.9%, p=0.07) and numerically lower two-year disabling stroke (Figure 1B). Moreover, it is of note that 30-day disabling stroke was lower with TAVI vs. SAVR (2.3% vs. 4.2%, p=0.04) in the transfemoral cohort, whereas no difference for this endpoint was observed among the transthoracic access cohort (6.0% vs. 4.5%, p=0.35).
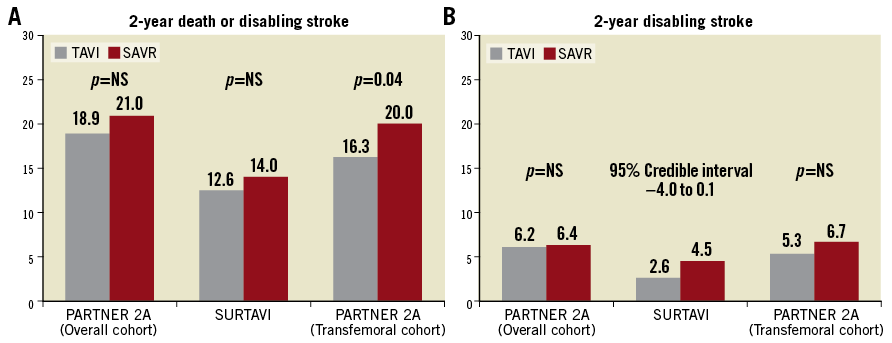
Figure 1. Rates of two-year events in PARTNER 2A and SURTAVI. A) All-cause death or disabling stroke. B) Disabling stroke.
The STACCATO trial compared TAVI with the SAPIEN valve by the transapical approach vs. SAVR in low-risk patients with isolated AS and age ≥75 years21. The study was prematurely terminated after the inclusion of 70 patients because of an excess of events in the TAVI group. The primary endpoint of 30-day all-cause mortality, stroke or renal failure was met in five (14.7%) patients in the transapical TAVI group vs. one (2.8%) patient in the SAVR group.
In summary, overall data available on TAVI vs. SAVR stratified according to the access route suggest a possible superiority only of transfemoral TAVI, but this hypothesis requires prospective evaluation in a suitably powered superiority study. Conversely, outcomes after transthoracic TAVI appeared to be inferior to those with transfemoral TAVI and were similar or worse than those with SAVR; however, further studies with emerging transthoracic access are needed to confirm these comparative findings.
Impact of TAVI versus SAVR trials
Two main conclusions can be summarised from the aforementioned trial results. First, across the risk spectrum from intermediate to high, TAVI seems to be at least on a par with SAVR in terms of mortality, although numerically lower deaths occurred in the TAVI vs. SAVR groups among higher-risk patients undergoing a transfemoral approach. Second, TAVI has been consistently associated with less AF, bleedings and renal injury, versus more access-site complications, PVL and PPI. Thus, overall, trials indicate a favourable benefit-risk balance for TAVI in a large risk spectrum, including relevant proportions of patients at lower levels of risk.
The overall trial results, along with consistent favourable data on TAVI registries22 and the observation of increasingly improved outcomes and reduced complications23, driven by newer TAVI devices, optimal procedural planning, simplified procedures and refined technique, have led to current guidelines upgrading and expanding TAVI indications. In particular, the 2017 American Guidelines24, released after PARTNER 2A and before the SURTAVI results, upgraded the indication for TAVI in inoperable and high-risk patients to Class IA and recommended for the first time that TAVI should be considered as an option in intermediate-risk patients (Class IIa B). The European Guidelines25, considering the overall surgical risk as a continuum with distinct extremes, stated that, in patients who are at increased surgical risk (STS or EuroSCORE II ≥4% or logistic EuroSCORE I ≥10% or other risk factors not included in these scores such as frailty, porcelain aorta, sequelae of chest radiation), the decision between SAVR and TAVI should be made by the Heart Team according to the individual patient characteristics, with TAVI being favoured in elderly patients suitable for transfemoral access (Class I B). The main patient-related factors that are suggested in favour of TAVI include the presence of severe comorbidities not adequately reflected by scores, age ≥75 years, previous cardiac surgery, frailty, restricted mobility and conditions that may affect the rehabilitation process after the procedure. In accordance with guideline and trial definitions24-26, a possible risk-assessment scheme combining mortality estimates by the STS score with factors augmenting the overall risk and potentially impacting on decision making is shown in Figure 2. Between the two extremes of risk (very low and very high) the assessment is a continuum of a progressively increasing risk scale with no perceptibly distinct limits (Figure 2).
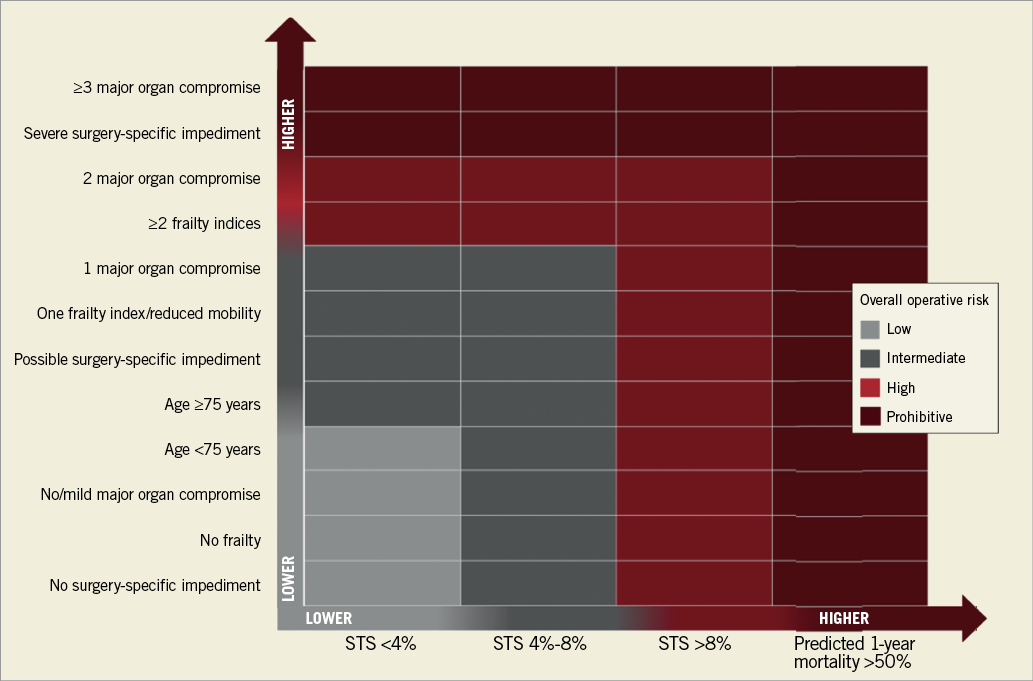
Figure 2. Risk assessment chart combining STS score with other clinical variables augmenting risk. Several scoring systems can be applied to calculate no, mild, or moderate-to-severe frailty. A simple assessment was suggested: no frailty (able to perform all activities of daily living and perform a five-metre walk in <6 seconds), mild degree of frailty (unable to perform one activity of daily living or unable to perform a five-metre walk in <6 seconds), and moderate-to-severe degree of frailty (unable to perform ≥2 activities of daily living). Examples of surgery-specific impediments: tracheostomy present, heavily calcified ascending aorta, chest malformation, arterial coronary graft adherent to posterior chest wall, or radiation damage. Classification of risk modified from Nishimura et al26.
Unresolved issues in TAVI versus SAVR trials
Despite strong recommendations supporting TAVI and the widespread use of this procedure, the following main issues remain from comparative trials of TAVI vs. SAVR: 1) the non-negligible overall risk of embolic neurological events; 2) the higher occurrences of PVL and new PPI; 3) the lack of systematic assessment of valve-leaflet thrombosis; 4) the relatively short follow-up, especially in trials on intermediate-risk patients, limiting the assessment of the bioprosthetic transcatheter valves’ long-term durability; 5) the lack of data on bicuspid aortic valves, which were an exclusion criterion of the described trials.
EMBOLIC NEUROLOGICAL EVENTS
Cerebrovascular accidents after TAVI significantly impair prognosis and are considered among the most serious complications occurring in the periprocedural period or in later phases27. In general, the incidence of all TAVI-related stroke decreases over time with 30-day mean rates as low as 2.5%, reflecting the lower-profile delivery systems and increased operator experience23. Despite this favourable trend, strategies such as cerebral embolic protection and anticoagulation, aiming to minimise the risk for overall embolic neurological events, are the focus of several ongoing TAVI randomised trials19,28. So far, studies assessing the impact of cerebral protection devices have provided inconclusive results. The largest, the SENTINEL trial, found that the use of the Sentinel™ dual-filter device (Claret Medical, Santa Rosa, CA, USA) was associated with a trend towards reduced new lesion volume and no difference in clinically manifest stroke or neurocognitive function29. A meta-analysis including this latter trial has shown that embolic protection devices were associated with a non-significant trend towards reduction in death or stroke30. Future trials will clarify the role of embolic protection devices during TAVI.
PARAVALVULAR LEAK
Residual moderate-to-severe PVL has been associated with reduced survival. Modifications of valve design and delivery system to prevent PVL have been a prominent focus in the development of the next-generation TAVI devices. The new-generation valves, along with the implementation of protocols for accurate valve size selection, have markedly reduced the incidences of PVL (0.2-7.5%) compared with those reported with first-generation valves (12-21%)23,31. The CHOICE randomised trial compared PVL rates (primary endpoint) between the early-generation self-expanding valve (CoreValve) and the second-generation balloon-expandable valve (SAPIEN XT) in 241 patients32. This trial showed that the rate of any degree of AR was lower in the balloon-expandable valve with less frequent moderate or severe AR (4% vs. 18%). Despite this, no differences in one-year clinical outcomes were observed in the trial, probably due to the limited statistical power. No comparisons are available between SAPIEN 3 and Evolut™ PRO (Medtronic), which have been associated with lower rates of PVL compared with their predecessor devices23. In the more recent REPRISE III trial33, the mechanically expanded, fully recapturable and repositionable LOTUS™ valve (Boston Scientific, Marlborough, MA, USA) implanted in 607 patients was associated with lower rates of one-year moderate-to-severe PVL compared with a self-expanding valve (CoreValve 51.5%, Evolut R 48.5%) implanted in 305 patients (0.9% vs. 6.9%, respectively p<0.001). However, the LOTUS valve had higher rates of new PPI (35.5% vs.19.6%; p<0.001). The REPRISE III study used the first-generation LOTUS, which has an implant mechanism involving significant interaction with the left ventricular outflow tract and conduction system. Thus, the valve is being iterated to reduce this interaction, thereby potentially lowering the PPI rate, but this will need to be tested in clinical trials. Indeed, several new-generation valves are available and are under clinical comparative assessment in ongoing trials comparing different TAVI prostheses19,28.
PERMANENT PACEMAKER IMPLANTATION
Pacemaker implantation has not been associated with decreased survival, at least in elderly patients, but it is associated with increased costs, longer hospital stay, and perhaps increased patient morbidity. While new valves have markedly reduced PVL, it appears that they are associated with increased occurrences of new PPI. In a recent meta-analysis including 40 studies (n=17,139), the incidence of PPI after the use of a new-generation TAVI prosthesis ranged between 2.3% and 36.1%34. Pre-existing conduction abnormalities (electrical factor), calcification of the left ventricular outflow tract (anatomical factor), and balloon valvuloplasty and depth of implantation (procedural factors) were associated with increased risk of PPI. Specific recommendations for implantation of each prosthesis, taking into consideration the presence of pre-existing conduction abnormalities and anatomical factors, may be needed to reduce the risk of PPI. Moreover, additional data on the time course of new-onset conduction abnormalities may help to refine the indication for PPI. Indeed, among patients receiving a pacemaker post TAVI, high rates of spontaneous resolution of conduction defects and relatively low rates of pacemaker dependency have been observed35.
VALVE THROMBOSIS
Reduced leaflet motion of bioprosthetic aortic valves caused by subclinical leaflet thrombosis has caught the attention of the cardiovascular community because of concerns regarding its potential clinical sequelae. Among 890 patients in the combined RESOLVE-SAVORY registries, 106 (12%) had subclinical leaflet thrombosis. Out of the 106, five (4%) were in the SAVR group and 101 (13%) were in the TAVI group36. Subclinical leaflet thrombosis resolved in all patients receiving anticoagulants. Subclinical leaflet thrombosis did not lead to higher stroke rates; however, increased rates of transient ischaemic attacks were observed. With regard to the question of a potentially different intrinsic thrombogenic activity of different valve systems, there is no clear evidence, but some signals have emerged in randomised trials. For instance, in the CHOICE trial higher numbers of possible valve thrombosis and stroke were observed in the SAPIEN XT group compared with the CoreValve group32. In the REPRISE III trial, the LOTUS valve was associated with higher rates of valve thrombosis but lower rates of stroke compared with CoreValve33. Thus, the overall clinical relevance of valve thrombosis and potential differences between devices remain to be adequately addressed. Overall, these results have prompted the need for more optimal antithrombotic therapy, which is a key topic currently being addressed in several ongoing TAVI trials19,28.
TAVI DURABILITY
Regarding valve durability, the five-year echocardiographic evaluations from PARTNER trials8 indicate no evidence of important premature or accelerated structural valve deterioration (SVD). In the NOTION trial, rates of five-year bioprosthetic valve failure, defined according to recent European standardised definitions, were similar between TAVR and surgery. Of note, SVD was noted to be substantially higher in the surgery group. No cases of thrombosis were noted in either group. However, longer-term follow-up is needed to assess valve durability accurately. Two recent registries have reported low rates (about 3%) of eight-year SVD37. However, further long-term studies are warranted.
BICUSPID AORTIC VALVE
No trial data are available on the safety and efficacy of TAVI in bicuspid valves, which have a high prevalence among patients <70 years of age38. Registries have shown that TAVI for bicuspid valves is associated with less favourable outcomes compared with those observed after TAVI in tricuspid valves39. However, outcomes in bicuspid valves are improving with new devices39. Thus, while bicuspid anatomy should not be considered a contraindication for TAVI, indications to this procedure must be carefully discussed based on patient-specific aortic root anatomy and calcium distribution. In the most recent guidelines, bicuspid aortic valve disease favours SAVR over TAVI25.
Cost-effectiveness of TAVI versus SAVR
Given the growing number of potential candidates for TAVI and the current high cost associated with this procedure, the evaluation of its economic effectiveness across the operative risk spectrum is of great importance to drive decisions on allocation of resources in a healthcare environment of increasing demands due to demographic and technology trends.
The cost-effectiveness analysis of the PARTNER 1B trial has shown that TAVI increases life expectancy (1.9 years over a lifetime horizon) at an incremental cost per life-year gained (US $50,200) that remained well within accepted values for cardiovascular technologies across a broad range of uncertainty and sensitivity analyses40. This finding was confirmed in several studies, which consistently reported that TAVI may be cost-effective in relation to medical therapy alone, as incremental cost-effectiveness ratios (ICERs) were in most cases close to or below maximum acceptable thresholds41.
In the PARTNER 1A trial-based economic analysis, only transfemoral TAVI provided a modest benefit in quality-adjusted life expectancy and slightly reduced costs compared with SAVR, resulting in an economically dominant strategy42. The difference favouring transfemoral TAVI vs. SAVR was driven by substantial reductions in length of stay, leading to cost savings sufficient to offset fully the higher TAVI procedural costs42. Consistent with PARTNER 1A results, the U.S. CoreValve trial showed that TAVI with a self-expanding prosthesis compared with SAVR provided meaningful survival benefits and acceptable ICERs, which could be markedly improved with a reduction in the initial cost of TAVI43. These economic findings in favour of TAVI in high-risk patients derived from trial-based analysis were not often confirmed in other studies using different modelling techniques and effectiveness sources and assessing costs from the perspective of several healthcare systems. This does not support a consistent and conclusive economic superiority of TAVI over SAVR in high-risk operable patients41. The inconsistency of overall economic studies applying different methods is multifactorial, but a major driver of cost differences between TAVI and SAVR across systems could have been the length of hospital stay.
Two recent cost-utility analyses using the PARTNER 2 and SURTAVI results as efficacy sources and applying costs from the perspective of the Canadian healthcare system have shown that TAVI provided favourable ICERs compared with SAVR in intermediate-risk populations44,45. However, in those analyses there was a moderate-to-high uncertainty, with the cost-effectiveness particularly sensitive to the cost of the TAVI prosthesis and the length of hospital stay. Thus, further studies are needed to assess the economic value of TAVI across countries, using new-generation valves and a more simplified approach, and across the overall operative risk spectrum. Also, residual uncertainty surrounding the very long-term outcomes of TAVI could have a substantive impact on its cost-effectiveness estimates.
Trials on percutaneous mitral valve repair
In current practice, PMVR is mainly performed with the MitraClip technique, which reproduces the surgical edge-to-edge leaflet repair by clipping together the free edges of valve leaflets at their mid portion. Other techniques for PMVR currently available for clinical use include coronary sinus annuloplasty with the Carillon® (Cardiac Dimensions, Kirkland, WA, USA) device or direct annuloplasty with a Cardioband (Edwards Lifesciences).
The clinical evidence on PMVR derives mostly from worldwide multicentre registries of high-risk or inoperable patients reporting high procedural success, good safety, and improved functional status and clinical symptoms after MitraClip procedures in patients with primary and secondary MR46-48. The Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) is the only randomised clinical trial to have compared PMVR with the MitraClip® (Abbott Vascular, Santa Clara, CA, USA) versus surgery in patients with chronic MR49,50. In the EVEREST II trial, 279 patients with grade 3+ or 4+ MR, mostly degenerative (73%) and with age <75 years (71%), were randomly assigned in a 2:1 ratio to undergo either percutaneous repair or conventional surgery for repair or replacement of the mitral valve. The primary efficacy endpoint of freedom from death, from surgery for mitral valve dysfunction, and from grade 3+ or 4+ MR at 12 months was significantly lower in the PMVR group compared with surgery (55% vs. 73%), driven by higher occurrence of surgery for mitral valve dysfunction in the PMVR group (20% vs. 2%, respectively). Severity of MR at 12 months and five years was significantly lower in the surgery group. No significant differences in mortality were observed between the two groups up to five years. After one year and up to five years, comparably low rates of surgery for mitral valve dysfunction with either percutaneous or surgical therapy were observed, endorsing the durability of MR reduction with both repair techniques. The EVEREST II trial included two prospective registries of high surgical risk patients undergoing MitraClip implantation: the EVEREST II High-Risk Registry (EVEREST II HRR) and the ongoing EVEREST II REALISM HR51. For both registries the definition of high risk was a surgical mortality risk of ≥12%, based on either the STS risk calculator or an estimate by the surgeon co-investigator following pre-specified protocol criteria including porcelain aorta, mobile ascending aortic atheroma, post-mediastinal radiation, functional MR with LVEF <40%, age ≥75 years with LVEF <40%, previous median sternotomy with patent bypass grafts, two previous chest surgeries, hepatic cirrhosis, or three of the following STS high-risk criteria: creatinine level >2.5 mg/dl, previous chest surgery, age ≥75 years, or LVEF <35%. Outcomes at one year of the EVEREST registries were reported for 351 patients with a mean age of 75.7±10.5 years, mostly with functional MR (70.1%) and 60% having prior cardiac surgery51. The MitraClip reduced MR to ≤2+ in 86% of patients at discharge and in 84% at 12 months, leading to significantly improved clinical symptoms and quality of life, and decreased LV dimensions. These benefits were associated with a short mean hospital stay of 3.2±4.9 days, and with low rates of complications and relatively low mortality. Overall, these efficacy and safety results are consistent with those achieved in several PMVR registries including secondary MR, which is currently the most common indication for MitraClip use in Europe. Non-randomised comparisons with historical controls have suggested that, in patients with severe secondary MR and left ventricular dysfunction, MitraClip might have a benefit (compared with medical therapy) in reducing the need for readmission to hospital and improving patient survival52. No randomised trials comparing survival with PMVR versus medical therapy in secondary MR are currently available.
Based on the available evidence on PMVR, current European and American guidelines on valvular heart disease indicate in Class IIb that MitraClip therapy may be considered in patients with symptomatic, severe, primary MR who fulfil the echocardiography criteria of eligibility and are judged inoperable or at high surgical risk by a Heart Team, avoiding futility25,26. Moreover, according to European guidelines25, PMVR could also be an option for patients with secondary MR, stating that MitraClip may be considered in patients at high surgical risk with no indication for revascularisation who remain symptomatic despite optimal medical management (including cardiac resynchronisation therapy if indicated) and who have a suitable valve morphology by echocardiography (Class IIb).
ONGOING TRIALS ON PERCUTANEOUS MITRAL VALVE REPAIR
The main design characteristics of ongoing trials comparing PMVR with surgery or with medical therapy are provided in Table 5.
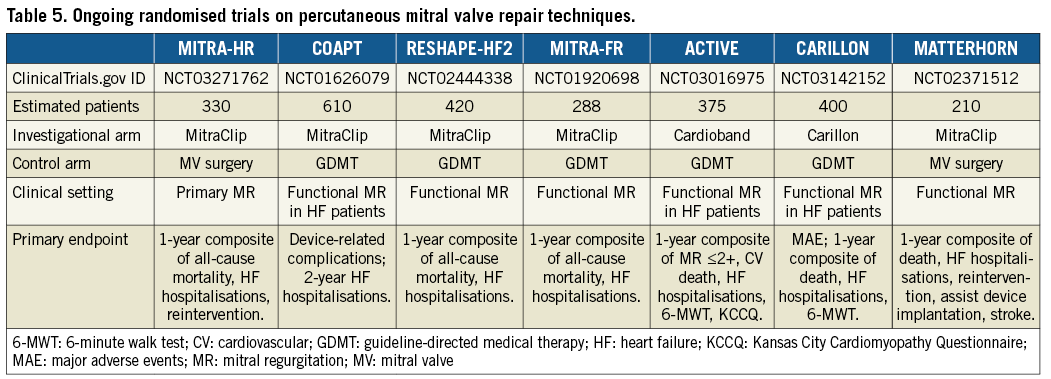
In the setting of primary MR, the MITRA-HR (Transcatheter Mitral Valve Repair in Patients With Severe Primary Mitral Regurgitation Eligible for High-risk Surgery) randomised trial will assess the non-inferiority of MitraClip versus surgery in terms of efficacy in patients with primary MR deemed at high surgical risk.
With regard to secondary MR, ongoing trials aim to assess the clinical efficacy of interrupting the dysfunctional cycle of volume overload from MR which causes more regurgitation. Indeed, several ongoing randomised trials (COAPT, RESHAPE-HF2, MITRA-FR) are comparing MitraClip versus optimal medical management only, including resynchronisation therapy, if indicated, by assessing the primary endpoint of death or readmission to hospital for heart failure at 12 months. Moreover, the ACTIVE (Annular ReduCtion for Transcatheter Treatment of Insufficient Mitral ValvE) and the CARILLON randomised trials are comparing PMVR using the Cardioband and Carillon systems, respectively, versus optimal medical therapy in patients with functional MR and heart failure. PMVR with the MitraClip will be compared with surgery in patients with functional MR in the MATTERHORN (Mitral vAlve reconsTrucTion for advancEd Insufficiency of Functional or iscHemic ORigiN) trial, assessing the primary composite endpoint of death, re-hospitalisation for heart failure, reintervention, assist device implantation and stroke at one year.
Conclusions
Randomised trials comparing TAVI vs. SAVR have led to significant advances in the field of transcatheter valvular interventions for the treatment of symptomatic severe AS. Indeed, those trials provided robust evidence for the expanded use of TAVI in patients at intermediate or higher operative risk. Moreover, these trials, along with evidence on increased TAVI efficacy and safety, have set strong foundations for future trials on new indications for TAVI (low risk, asymptomatic severe AS, heart failure and moderate AS). Some residual issues concerning TAVI, which can be of crucial importance among low-risk patients, are the focus of dedicated studies, device iterations and procedural refinements. In the mitral field several ongoing trials comparing PMVR with medical therapy or surgery are eagerly awaited and will help to define optimal MR management in this era of evolving catheter-based treatment options.
Conflict of interest statement
N. Van Mieghem has received research grant support from Boston Scientific, Edwards Lifesciences, Medtronic, St. Jude Medical, Abbott Vascular, and Claret Medical. C. Tamburino has received honoraria/lecture fees from Abbott Vascular and Medtronic. P. Capranzano has no conflicts of interest to declare.

