
Introduction to the session: the trial headlines
The aim of this article is to capture the session at EuroPCR 2017 covering the SURTAVI study, communicate the analysis of the trialists, and report the views expressed in the interactive discussion. This article does not constitute an independent review of the topic by the authors.
The SURTAVI study (Safety and Efficacy of the CoreValve® System in the Treatment of Severe, Symptomatic Aortic Stenosis in Intermediate Risk Subjects Who Need Aortic Valve Replacement)1 was the focus of the discussion during the dedicated session at EuroPCR 2017 entitled “Will this trial change my practice?”. The session was chaired by Neil Moat (UK) and co-chaired by Didier Tchétché (France). The remaining invited panellists were all active interventional/structural cardiologists with extensive experience in conducting clinical research. John Forrest (USA) presented a clinical case during the session to illustrate the clinical challenge of selecting treatment for an intermediate-risk patient with severe aortic stenosis (AS). Farrel Hellig (South Africa) outlined what was already known from the literature before the SURTAVI study, whilst Alexandra Lansky (USA) provided insightful perspectives as an experienced trialist on the methods, results and conclusion of the SURTAVI study. The session was rounded off by John Forrest who revealed how he had in fact treated his patient, and the session was concluded with some closing remarks and key take-home messages by Neil Moat.
The session was introduced by the co-chair (Didier Tchétché) who led an expert panel through an initial review of the major results of the SURTAVI trial and discussed their relevance in everyday practice. He summarised the main trial findings, which demonstrated that transcatheter aortic valve implantation (TAVI) with the self-expanding CoreValve® system (Medtronic, Minneapolis, MN, USA) was non-inferior to surgical aortic valve replacement (SAVR) over 24-month follow-up with respect to the primary endpoint (mortality/stroke). The key learning objectives of the session were to review the results of SURTAVI, to learn how patient selection defines outcomes in TAVI and SAVR and to relate the findings of the trial to everyday practice.
Case presentation: how should I treat?
A case was presented by John Forrest, which clearly illustrated the clinical challenge in selecting the correct treatment for a patient with severe AS of intermediate surgical risk. The case described was that of a 78-year-old female with several comorbidities (previous percutaneous coronary intervention [PCI] to the left anterior descending [LAD] artery following a non-ST-elevation myocardial infarction three years earlier), type 2 diabetes mellitus (DM), chronic kidney disease (CKD: creatinine 1.1), asthma and a high body mass index (BMI) (35) who had severe AS (mean gradient 66 mmHg, aortic valve area [AVA] 0.58 cm²) with preserved left ventricular (LV) function (left ventricular ejection fraction [LVEF] 72%). Her NYHA Class was III but she was generally independent in activities of daily living (ADLs). She had self-referred for consideration of TAVI having been told she should undergo SAVR following an admission with heart failure. She was undecided and keen to discuss her options. Work-up for TAVI revealed a forced expiratory volume (FEV1) of 78% predicted, a patent LAD stent, with otherwise mild coronary atheroma, aortic annular dimensions of 19 by 26 mm (mean 22 mm) and a heavily calcified aortic valve (AVA 367 mm2). Her calculated STS score was 4.3.
“What is common practice?” discussion
The panel discussed the case, highlighting that, interestingly, although the patient’s surgical STS score was intermediate, the data presented appeared on face value to suggest a higher than intermediate-risk patient (high BMI, CKD, type 2 DM), meaning that in current practice TAVI and SAVR could be treatment options. Michael Reardon (the SURTAVI principal investigator) present in the audience felt that the patient would have been suitable for the trial with the whole panel agreeing that risk scores were not absolute and that it is important especially in a case of intermediate risk to see and discuss the options with the patient. It was generally agreed that in current European practice the patient would have been suitbale for TAVI.
Background: what was known before the trial?
Farrel Hellig reviewed what was known about TAVI compared to conventional SAVR prior to the SURTAVI trial. He covered the randomised controlled trials (RCT) performed to date including PARTNER high risk2 (mean age 84, STS 12%), Pivotal3 (age 83, STS 7.5%), NOTION4 (age 79, STS 3%), PARTNER 2A5 (age 81.5, STS 6%), all of which demonstrated non-inferiority of TAVI to SAVR in higher-risk patients. A meta-analysis of RCT suggests the potential superiority of TAVI compared to SAVR in these higher-risk patient groups6, with the importance of both risk score and age emphasised. Issues with TAVI in these trials were discussed (need for permanent pacemaker [PPM] insertion, vascular access complications and paravalvular leaks [PVL]) and the potential that newer second-generation devices may mitigate against these problems - issues that may be all the more important in lower-risk individuals with respect to long-term TAVI efficacy.
Trial analysis: summary of the trialists’ critical review
Alexandra Lansky reviewed the SURTAVI trial design and outcomes1. The trial was designed to evaluate the safety and efficacy of TAVI with CoreValve vs. SAVR in symptomatic severe AS patients at intermediate surgical risk (STS score 3-15%). Participants were randomised to TAVI or SAVR following Heart Team discussion regarding suitability. The primary endpoint was all-cause death or disabling stroke at 24 months of follow-up. Secondary endpoints included aortic valve reintervention, major vascular complications, life-threatening or major bleeding, pacemaker implantation, major adverse cardiovascular and cerebrovascular events (MACCE) and patient quality of life (assessed by the Kansas City Cardiomyopathy Questionnaire [KCCQ]). The global trial ran between 2012 and 2016 at 87 sites worldwide (USA 65, Canada 5, Europe 17), and in the opinion of Alexandra Lansky (and the panel) had exemplary trial leadership and oversight. Alexandra Lansky highlighted that the majority of the 863 patients in the TAVI group were treated with a first-generation valve with only 139 treated with the second-generation Evolut™ R (Medtronic), highlighting the potential for further improvement with the newer device. The thorough stroke assessment, which formed part of the primary endpoint, was complimented, as was the elegant but complicated statistical design (Bayesian analysis). The patient population was one of truly intermediate risk (age 80, mean STS score 4.5%) with well-balanced frailty indices. Primary analysis was a modified intention-to-treat analysis (including all those with an attempted procedure). Despite the SAVR arm performing exceptionally well, with a low observed to expected surgical mortality (original assumption 17% event rate, actual rate 14%), SURTAVI met its primary endpoint demonstrating that TAVI with CoreValve and Evolut R systems is non-inferior to SAVR for a combined endpoint of all-cause mortality or disabling stroke at 24 months (Figure 1). At 30 days, TAVI was associated with a significantly lower rate of all stroke, acute kidney injury and atrial fibrillation, while SAVR had lower rates of PVL, major vascular complications and new permanent pacemaker. TAVI resulted in better haemodynamics with significantly lower mean gradients and greater AVAs than SAVR at all post-implant time points.
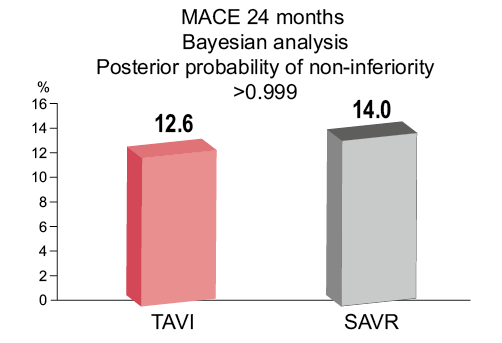
Figure 1. TAVI was non-inferior to SAVR with respect to all-cause mortality and stroke at 24 months. Figure reproduced from the PCR Trials Book.
“Will this trial change my practice?” discussion
Aspects of the trial were discussed amongst the panel. It was summarised that, although TAVI was non-inferior to SAVR, a trade-off between lower stroke rates, better haemodynamics and reduced acute kidney injury (AKI) had to be made against higher PPM and vascular complications, although both of these may decline with improved devices. The issue of higher stroke rates in the SAVR group, which may relate to the higher rates of atrial fibrillation (AF) seen post SAVR, was debated and the importance of this endpoint from a patient perspective emphasised. The issue of PVL was hotly debated with it being highlighted that, as TAVIs are utilised in lower-risk patient groups which have a greater life expectancy, PVL and any potential future consequences become increasingly important. The long-term effects of PVL post TAVI are unknown, despite the reassurance of no adverse effects seen in the follow-up period of SURTAVI. This led on to the conclusion of the case, for which the main pre-procedure concern had been the risk of PVL.
John Forrest presented the conclusion to his case, demonstrating that the patient had been successfully treated by TAVI. Post initial 26 mm Evolut deployment, there was a moderate PVL related to the localised heavy aortic calcification. This had been the main concern pre-procedure, and the procedure had been performed under general anaesthesia (GA) with transoesophageal (TOE) guidance as a precaution. This leak resolved after post-dilatation and the patient was discharged on day two with only trivial PVL at 30-day follow-up. The recent focused update to the American guidelines (AHA/ACC)7 was discussed with TAVI now a class IIa indication; however, this change was prior to publication of the trial and the panel all agreed that perhaps following SURTAVI a further update was required.
The session ended with a lively discussion between the panellists and the audience as to whether or not this trial will/should change our clinical practice. It was highlighted that, although the results in intermediate surgical risk patients were promising, data concerning longer-term outcomes from TAVI in this patient group were still lacking. Patient preference and individual patient factors, such as age and small left ventricular outflow tract, need to be considered and accounted for in addition to simple surgical risk. It was wholeheartedly agreed that the introduction of TAVI has started to address the unmet need of untreated severe AS with Michael Reardon highlighting that soon TAVI will outnumber SAVR in the USA; however, more needs to be done.
The Chairperson’s conclusion: where do we stand now?
The session was completed with a summary from Neil Moat during which it was agreed that SURTAVI has demonstrated that excellent clinical outcomes can be achieved with either SAVR or TAVI and that TAVI now appears an appropriate alternative in an intermediate-risk (STS 4-6%) population.
| Summary ARGUMENTS FOR A CHANGE IN PRACTICE – Two large multicentre trials (SURTAVI and PARTNER 2A) have now shown non-inferiority of TAVI versus SAVR for treatment of patients with severe AS at intermediate surgical risk. – SURTAVI showed that TAVI was associated with better haemodynamics, a significantly lower rate of all stroke at 30 days, acute kidney injury and atrial fibrillation compared to SAVR. ARGUMENTS AGAINST A CHANGE IN PRACTICE – The long-term durability and effects of TAVI are still under investigation. – SAVR continues to have lower rates of residual PVL, major vascular complications and new permanent pacemakers compared to TAVI, although newer TAVI devices may change this. |
Conflict of interest statement
N. Moat has received speaker and consultant fees from Medtronic, Abbott, and Edwards. D. Tchétché is a consultant for Medtronic. J. Forrest has received grant support and consulting fees from Edwards Lifesciences and Medtronic. The other authors have no conflicts of interest to declare.

