
Introduction to the session
The aim of the article is to capture the session at EuroPCR 2016, communicate the analysis of the trialist, and report the views expressed in the interactive discussion. The article does not constitute an independent review of the topic by the authors. The session focused on whether the PARTNER 2 Cohort A trial will change clinical practice1.
Introduction
The introductory comments highlighted that the indications for TAVI in 2016 are “frozen in time”, being based upon ESC and ACC/AHA guidelines published in 2012 and 2014, respectively. The pivotal PARTNER 1 trial demonstrated the utility of TAVI in inoperable and high-risk groups and underpinned these guidelines which recommend TAVI in patients with severe aortic stenosis who are at high surgical risk (Class IIa, Level B) or inoperable (Class I, Level B). The PARTNER 2 Cohort A trial1 sought to compare the outcomes of TAVI using a second-generation valve (SAPIEN XT; Edwards Lifesciences, CA, USA) and surgical AVR (SAVR) in an intermediate-risk group (STS score >4).
Three case examples illustrated typical intermediate-risk patients enrolled in the trial: 1) an 86-year-old male with prior coronary artery bypass grafting (STS score 4.66%), 2) a 78-year-old male with multivessel coronary artery disease and non-Hodgkin’s lymphoma (STS score 4.67%), and 3) a 76-year-old female with morbid obesity (BMI 39.6) and multiple comorbidities whose participation in the trial was advocated by her treating cardiologist despite a lower STS score (3.23%).
Background: what was known before the PARTNER 2 Cohort A trial
The audience was presented with an overview of the increasing number of TAVI procedures being performed in the United Kingdom and Germany. The number of cases has risen steadily in both countries and the annual number of TAVI procedures in Germany (n=13,264 in 2014) now exceeds SAVR (with and without coronary artery bypass grafting) with an accompanying progressive reduction of in-hospital mortality and major complications. A clear summary was provided of the pre-existing robust data confirming the role of TAVI in high-risk and inoperable patients. PARTNER 1 (Cohort B)2 established that TAVI reduced mortality in comparison with medical therapy (number needed to treat = 5) while PARTNER 1 (Cohort A)3 confirmed equivalent outcomes of TAVI and SAVR in high-risk groups at two-year follow-up. Moreover, the CoreValve High-Risk trial4 confirmed a trend observed in more recent studies, with transfemoral TAVI surpassing the results of SAVR. Taken together, these trials demonstrated the non-inferiority of TAVI compared with SAVR in high-risk patients and inferred that transfemoral TAVI was associated with superior outcomes. A progressive reduction in mortality was also observed in the lower-risk subgroup (STS score ≤7%) of the CoreValve Pivotal trial5, whilst the NOTION trial6, which compared TAVI and SAVR in low-risk patients (81.8% of participants STS score ≤4%), demonstrated a trend towards reduced mortality in the TAVI cohort, consistent with other trials. Hence, TAVI results have been consistent across different trials involving a variety of transcatheter devices.
The potential reasons for the progressive improvement in TAVI outcomes were then discussed. First, refinements in valve design have significantly reduced the rate of post-implantation paravalvular regurgitation. Second, with increasing operator experience, TAVI devices are currently deployed more accurately compared to initial experiences. This, along with careful patient selection, has reduced permanent pacemaker requirements (which may fall further in intermediate- and low-risk groups with intact conducting systems). Third, there has been a dramatic increase in the proportion of TAVI procedures undertaken using a fully percutaneous transfemoral approach in recent years, reflecting increasing operator experience and the availability of lower-profile delivery systems. Fourth, more centres are adopting conscious sedation protocols, thereby avoiding the potential complications of general anaesthesia and facilitating earlier hospital discharge.
Forces driving the move to perform TAVI in an intermediate-risk group were then addressed. As the population ages, there is an increasing patient population in need - with increasing awareness of aortic stenosis as a condition and TAVI as a potential line of treatment, referrals to Heart Teams for consideration of TAVI will inevitably increase. This direction of travel seems to be broadly accepted by cardiothoracic surgeons, and patients prefer the percutaneous approach rather than a sternotomy. Furthermore, the combination of improved efficiency (allowing three or more procedures per day in most units), reduced personnel required for the procedure and shorter length of stay, seems destined to reduce overall procedural costs. A more minimalist approach to TAVI has been proven to drive cost savings without detrimental effects to patients. Hence, TAVI is likely to prove a more cost-effective treatment option than SAVR.
In summary, TAVI is becoming simpler and safer. The procedure is non-inferior to SAVR in high-risk groups and there is a trend towards reduced mortality with transfemoral TAVI and in lower-risk groups. Newer devices are associated with reduced rates of paravalvular regurgitation, and increasing operator experience has resulted in a better understanding of the pacemaker need. Shorter length of stay will influence cost-effectiveness, and patients are likely to prefer a minimally invasive approach. These are the forces driving evaluation of TAVI in intermediate-risk groups.
Trial analysis: summary of the trialist’s critical review
PARTNER 2 Cohort A is a randomised controlled trial comparing TAVI and SAVR in 2,032 intermediate-risk patients with symptomatic severe aortic stenosis (aortic valve area ≤0.8 cm2 or <0.5 cm2/m2 body surface area with mean aortic valve gradient >40 mmHg or peak jet velocity >4.0 m/s). Participants were assessed by a Heart Team and included in the study if they were operable with an STS score ≥4%. Then they were stratified according to the feasibility of transfemoral access and randomised 1:1 to TAVI or SAVR. The primary endpoint was a composite of all-cause mortality or disabling stroke at two years.
Before outlining the trial outcomes, some key design points were raised. First, similar to PARTNER 1, this trial was conducted in multiple sites across the United States. Importantly, these centres had overcome their original procedural learning curves which they had accumulated during PARTNER 1 and ensuing clinical practice. Second, this trial, though important for the formulation of future guidelines in providing level A evidence, is already somewhat outdated on account of the rapid maturity of TAVI and use of a second-generation TAVI device, the SAPIEN XT valve. The newer SAPIEN 3 valve incorporates a circumferential skirt which virtually eliminates the issue of paravalvular regurgitation following implantation. Third, the mean age of enrolled patients was 82 years with a mean STS score of 5.8% in both arms – not dissimilar to other trials such as the CoreValve High-Risk study (mean age 83 years, mean STS score 7.4%) and NOTION (mean age 79 years, mean STS score 3%). Therefore, although the PARTNER 2 Cohort A was billed as an intermediate-risk trial, it is largely reflective of current practice in Europe and overlaps with the pre-existing evidence base.
The primary endpoint of all-cause mortality or disabling stroke at two years was similar in the TAVI and SAVR study groups (19.3% vs. 21.1%, p=0.25). Importantly, a predefined sub-analysis demonstrated a clear advantage of transfemoral TAVI over SAVR (primary endpoint 16.8% vs. 20.4%, p=0.05) with favourable implications for contemporary practice where 90% of TAVI using the SAPIEN 3 valve are performed via the transfemoral approach (Figure 1).
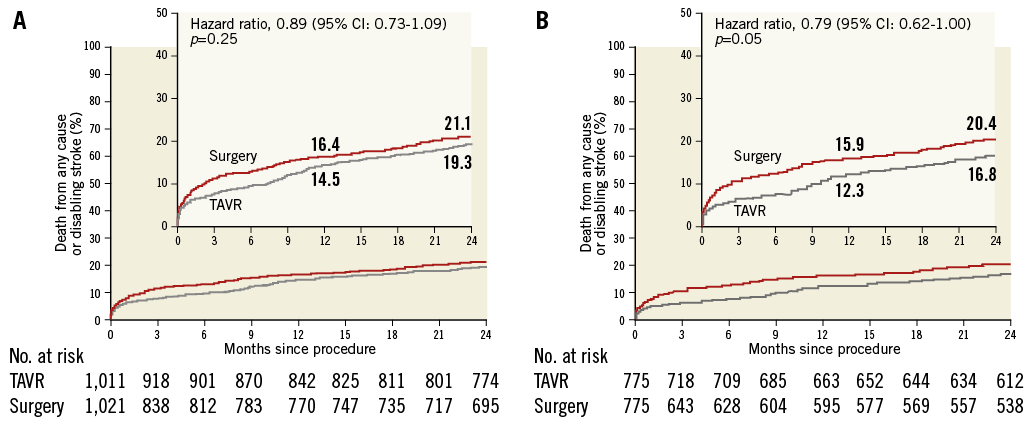
Figure 1. Primary endpoint analysis. A) Intention-to-treat population. B) Transfemoral access cohort, intention-to-treat analysis.
Other important clinical endpoints were discussed. Although the rate of major vascular complications was slightly higher after TAVI than SAVR at 30 days (7.9% vs. 5%, p=0.008), this was offset by a much higher incidence of life-threatening/disabling bleeding after SAVR (43.4% vs. 10.4%, p<0.001). Furthermore, renal impairment (1.3% vs. 3.1%, p=0.006) and new-onset atrial fibrillation (9.1% vs. 26.4%, p<0.001) were less frequent in the TAVI group, while rates of permanent pacemaker requirement were equivalent (8.5% vs. 6.9%, p=NS). Reassuringly, there was no difference in the rates of second intervention and no incidence of valve-related endocarditis in either arm.
Echocardiographic studies demonstrated sustained valve function and superior performance of the TAVI valves at 30-day and two-year follow-up (valve area 1.68 cm² vs. 1.47 cm² and 1.54 cm² vs. 1.4 cm², respectively, both p<0.001). Over longer-term follow-up, these differences may prove important in terms of prosthetic durability and sustained relief of symptoms, particularly when TAVI is driven towards lower-risk patients and used in younger, female patients with a smaller body surface area who are particularly at risk for patient-prosthesis mismatch.
Finally, the study provided reassuring information concerning the clinical significance of residual mild paravalvular regurgitation after TAVI. Thus, whilst earlier trials had identified a signal suggesting that mild paravalvular regurgitation was associated with poor outcome over long-term follow-up (the so-called “Achilles heel of TAVI”), outcomes in subjects with mild paravalvular regurgitation after TAVI in PARTNER 2 Cohort A matched those in subjects with no/trace regurgitation. Although those with moderate or severe paravalvular regurgitation fared less well, this finding was rare (8%) and has now been virtually abolished with contemporary TAVI devices. Indeed, in the parallel PARTNER S3i study using the third-generation SAPIEN 3 device7, rates of mortality, stroke and at least moderate paravalvular regurgitation were remarkably low at one year (7.4%, 4.6% and 1.5%, respectively) and superior to propensity-matched controls undergoing SAVR in the PARTNER 2 Cohort A.
The Chairperson’s conclusion: where do we stand now?
The session was concluded by reiterating that the favourable results of TAVI have been reproducible across different trials in different patient subsets using a variety of TAVI devices over time. Although newer trials have incorporated patients with lower STS scores and surgical risk, their average age has remained similar at 80 years of age, consistent with current European practice. Demonstrating the role of TAVI in even younger and lower-risk subsets remains an important priority that will be addressed by the forthcoming NOTION 2 and PARTNER 3 studies.
Meanwhile, the results of the PARTNER 2 Cohort A trial seem set to change everyday practice, encouraging the use of TAVI as an alternative to SAVR in intermediate risk subjects and providing the robust Level 1A evidence required for modification of international guidelines to endorse wider and more liberal use of TAVI in symptomatic severe aortic stenosis.
Conflict of interest statement
R. Makkar has received research grants from Edwards Lifesciences, Medtronic and St. Jude Medical. P. MacCarthy is a proctor for Edwards Lifesciences. L. Sondergaard has received research grants from Medtronic, St. Jude Medical, Boston Scientific and Symetis and is a proctor for Medtronic, St. Jude Medical and Boston Scientific. H. Eltchaninoff is a proctor for Edwards Lifesciences. B. Prendergast has received lecture fees from Edwards Lifesciences and Boston Scientific. D. Natarajan has no conflict of interest to declare.

