Abstract
Transcatheter aortic valve implantation (TAVI) has become the preferred treatment option for patients with severe aortic stenosis at increased risk for surgical aortic valve replacement (SAVR) and for older patients irrespective of risk. However, in younger, low-risk patients for whom both therapeutic options, TAVI and SAVR, are applicable, the optimal treatment strategy remains controversial, as data on long-term outcomes remain limited. The DEDICATE-DZHK6 Trial is an investigator-initiated, industry-independent, prospective, multicentre, randomised controlled trial investigating the efficacy and safety of TAVI compared to SAVR in low- to intermediate-risk patients aged 65 years or older. To evaluate both treatment strategies, approximately 1,404 patients determined eligible for both TAVI and SAVR by the interdisciplinary Heart Team were randomised to TAVI or SAVR. Broad inclusion and strict exclusion criteria targeted an all-comers patient population. Procedures were performed according to local best practice with contemporary routine medical devices. The primary endpoints are a composite of mortality or stroke at 1 year and 5 years in order to incorporate midterm efficacy results and complement early safety data. Primary outcomes will be tested sequentially for non-inferiority and superiority. The DEDICATE-DZHK6 Trial has been designed to mirror clinical reality for the treatment of severe aortic stenosis and provide unique information on overall outcomes after TAVI and SAVR that can be directly applied to clinical routines. Its results will help further define optimal treatment strategies for low- to intermediate-risk patients in whom both TAVI and SAVR are currently advisable.
Introduction
Transcatheter aortic valve implantation (TAVI) has become the preferred treatment option for patients with symptomatic severe aortic stenosis at increased operative risk across all age groups and for older patients, irrespective of operative risk, if a transfemoral approach is feasible123. In younger patients for whom both therapeutic options, TAVI and surgical aortic valve replacement (SAVR), are applicable, the optimal treatment strategy remains controversial. As a response to the recently published low-risk trials4567, TAVI has been expanded towards this patient population. In the absence of long-term results and robust durability data for the medical devices, guidelines emphasise an individualised Heart Team approach for treatment selection138. The limitations of published evidence particularly relate to strict patient selection, composite primary outcomes limited to short-term follow-up and restrictions to specific transcatheter heart valve devices. We therefore designed an investigator-initiated, industry-independent, prospective, multicentre, randomised controlled trial (RCT) − the DEDICATE-DZHK6 Trial − for comparing TAVI with SAVR. In this trial, we aim to demonstrate the non-inferiority of TAVI versus SAVR at 1 and 5 years for the co-primary safety endpoints; if non-inferiority is demonstrated, we will subsequently test for superiority for the 5-year primary clinical efficacy endpoint. As the treatment strategies are being compared, SAVR or TAVI were performed according to local best practice, and all contemporary routine medical devices were allowed in both treatment strata. The trial was designed so that the patient population mirrors the clinical reality for the treatment of severe symptomatic aortic stenosis in Germany at the time of study inclusion.
Methods
Rationale and trial design
DEDICATE-DZHK6 (Randomized, Multi-Center, Event-Driven Trial of TAVI versus SAVR in Patients with Symptomatic Severe Aortic Valve Stenosis and Intermediate Risk of Mortality, as Assessed by STS-Score; ClinicalTrials.gov: NCT03112980; date of registration: 13 April 2017) is an RCT designed to assess the safety and efficacy of TAVI compared to SAVR in the treatment of patients with symptomatic severe aortic stenosis at low to intermediate operative risk of mortality. The lead Hamburg Ethics Committee (reference number PV5417) and the local ethics committees at the participating study sites approved the study protocol. The study flow is depicted in Figure 1, and the participating centres are listed in Supplementary Table 1. An independent data safety and monitoring board is responsible for monitoring patient safety and evaluating the efficacy and conduct of the study. All boards and committees are listed in Supplementary Table 2.
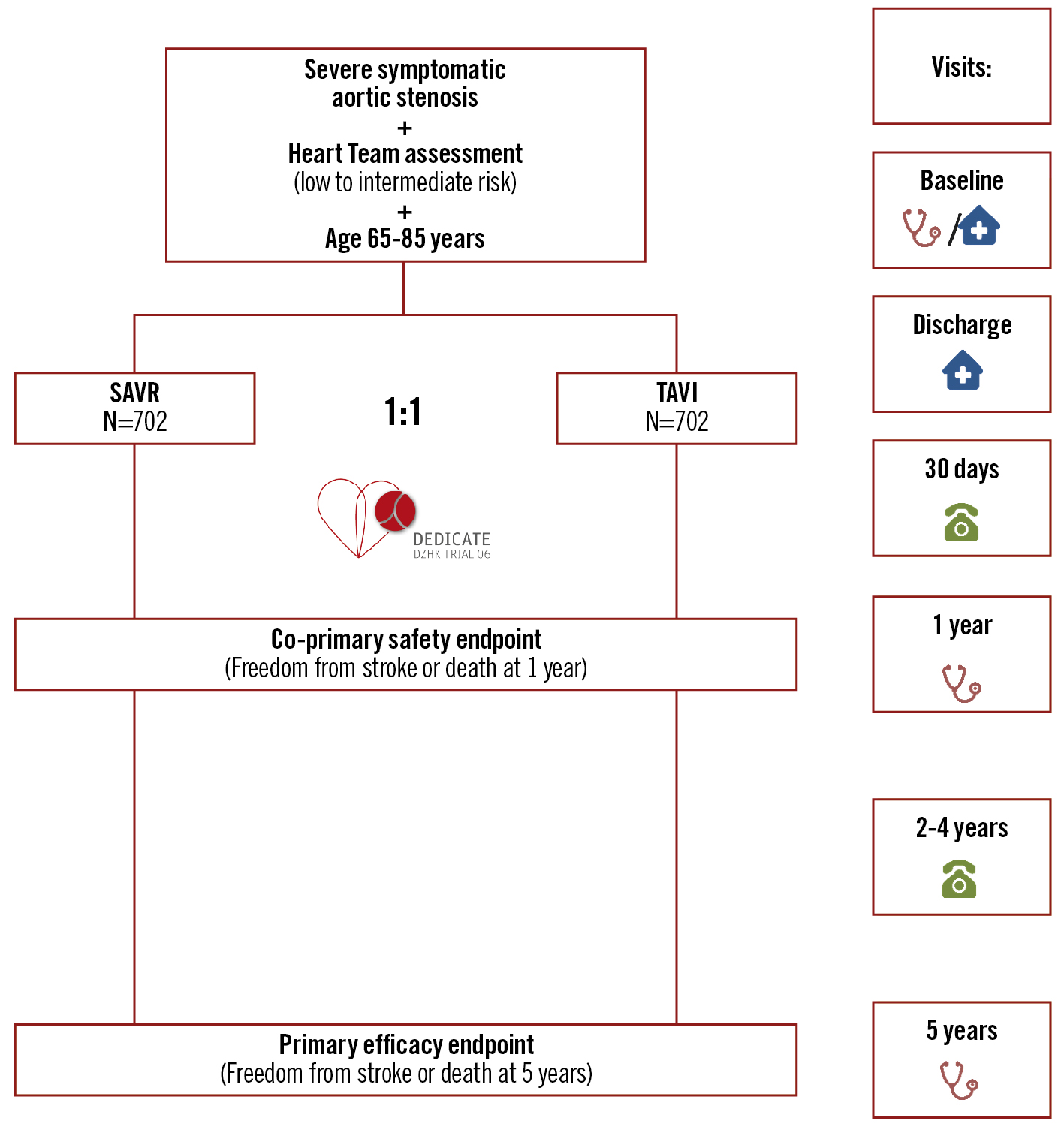
Figure 1. Study flowchart of the DEDICATE-DZHK6 Trial. Enrolled patients are randomised in a 1:1 ratio to isolated surgical aortic valve replacement (SAVR) or isolated transcatheter aortic valve implantation (TAVI).
Eligibility and screening
Low- to intermediate-risk patients with severe symptomatic tricuspid aortic stenosis in whom both isolated SAVR or isolated TAVI were advisable, according to Heart Team consensus, were screened for enrolment into the trial. To maximise generalisability and representativeness, we applied broad inclusion criteria and strict exclusion criteria (Table 1). As both medical practice and the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) calculation evolved during the recruitment phase, the initial STS-PROM cut-off value was waived, and a lower age limit of 65 years was implemented. This also took into account that current risk stratification tools performed poorly in estimating outcomes after TAVI, yielding a pragmatic Heart Team-centred screening process. Enrolment started in May 2017 and was completed in September 2022.
Table 1. Eligibility criteria.
| Inclusion criteria |
|---|
| 1. Heart Team consensus that isolated TAVI and SAVR are both medically justified and advisable based on |
| (a) degenerative aortic valve stenosis with echocardiographically derived criteria (mean gradient >40 mmHg OR jet velocity greater than 4.0 m/s OR aortic valve area [AVA] of <1.0 cm2 [indexed EOA <0.6 cm2/m2]) |
| (b) patient symptomatic from his/her aortic valve stenosis (NYHA Functional Class ≥II OR angina pectoris OR syncope) |
| (c) patient classified as low to intermediate operative risk as assessed by the local Heart Team according to variables outlined in the 2017 ESC/EACTS Guidelines for Management of Valvular Heart Disease, taking into account cardiac and extracardiac patient characteristics and established risk scores (e.g., STS-PROM, EuroSCORE) |
| (d) transfemoral or alternative access for TAVI seems feasible; centres should follow a “transfemoral first” strategy for primary route of access; however, other routes of access are also allowed, as decided by local Heart Team consensus |
| 2. Patient aged 65-85 years |
| 3. Patient provided written informed consent to participate in the trial |
| 4. Ability of patient to understand patient information and to personally sign and date informed consent to participate in study, before performing any study-related procedures |
| 5. Patient agrees to undergo SAVR, if randomised to control treatment |
| 6. Patient and treating physician agree that patient will return for all required postprocedural follow-up visits |
| 7. Male gender or postmenopausal (defined as no menses for 12 months without an alternative medical cause) in case of female gender |
| Exclusion criteria |
| 1. Aortic valve is congenital unicuspid or congenital bicuspid valve, or non-calcified |
| 2. Untreated clinically significant coronary artery disease considered a contraindication to isolated aortic valve procedure (TAVI or SAVR) according to Heart Team consensus |
| 3. Any percutaneous coronary intervention performed within 1 month prior to study procedure |
| 4. Prior cardiac surgery |
| 5. Untreated severe mitral or tricuspid regurgitation |
| 6. Untreated severe mitral stenosis |
| 7. Haemodynamic instability requiring inotropic support or mechanical circulatory support |
| 8. Ischaemic stroke or intracranial bleeding within 1 month |
| 9. Severe ventricular dysfunction with left ventricular ejection fraction <20% as measured by resting echocardiogram |
| 10. Hypertrophic obstructive cardiomyopathy or severe basal septal hypertrophy with outflow gradient |
| 11. Echocardiographic evidence of intracardiac mass, thrombus, vegetation or endocarditis |
| 12. Any other condition considered a contraindication for an isolated aortic valve procedure |
| 13. Symptomatic carotid or vertebral artery disease |
| 14. Expected life expectancy <12 months due to associated non-cardiac comorbidities |
| 15. Currently participating in another investigational drug or device trial |
| EACTS: European Association for Cardiac and Thoracic Surgery; EOA: effective orifice area; ESC: European Society of Cardiology; EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association; SAVR: surgical aortic valve replacement; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI: transcatheter aortic valve implantation |
Randomisation, treatment, and follow-up
After informed consent was obtained, patients were randomised in a 1:1 ratio to TAVI or SAVR using a balanced stratified block randomisation with variable block lengths, stratified by STS-PROM (0-2.00%, 2.01-4.00%, 4.01-6.00%) and study site. Randomisation was performed using the validated randomisation software RITA9 within the electronic case report forms.
The assigned treatment (TAVI or SAVR) was performed following treatment guidelines and according to local best practice12. The choice of the respective valve prosthesis, the access site, and other (peri)procedural aspects were left to the discretion of the implant team in order to mirror clinical reality and prevent a potential device-based bias. Procedures were performed in accordance with the recommendations of the “Gemeinsamer Bundesausschuss” (Federal Joint Committee, which determines the list of benefits provided by statutory health insurance) for minimally invasive heart valve procedures in Germany. Patients will be followed up for at least 5 years after randomisation, with scheduled telephone visits at 30 days, 2, 3, and 4 years and with scheduled outpatient visits at 1 and 5 years (Figure 1). Clinical status, clinical events, quality-of-life questionnaires (EQ-5D), electrocardiograms (ECG), and echocardiographic and laboratory data, among other data − see protocol (Supplementary Appendix 1), will be obtained. Echocardiographic and computed tomography examinations will be independently assessed by core laboratories to validate findings and increase data quality.
Study endpoints
The co-primary safety endpoint, the primary efficacy endpoint and secondary endpoints are listed in Table 2. Outcome measures are defined in accordance with the updated Valve Academic Research Consortium (VARC)-2 consensus document10, as this was the most current consensus document at the time of the study design and first enrolment. Endpoints are adjudicated in a blinded fashion by an independent event adjudication committee.
Table 2. Primary and major secondary endpoints.
| Primary efficacy endpoint |
|---|
| Freedom from stroke or death within 5 years after randomisation |
| Co-primary safety endpoint |
| Freedom from stroke or death within 1 year after randomisation |
| Secondary endpoints |
| Overall survival |
| Freedom from stroke or death |
| Freedom from cardiovascular mortality |
| Freedom from myocardial infarction |
| Freedom from stroke |
| Freedom from major or life-threatening/disabling bleeding |
| Freedom from acute kidney injury |
| Freedom from major vascular access site and access-related complications |
| Freedom from conduction disturbances and arrhythmias, need for permanent pacemaker implantation |
| Freedom from prosthetic valve dysfunction |
| Freedom from prosthetic aortic valve endocarditis |
| Freedom from (re)hospitalisation |
| Quality-of-life measures (improvement in quality-of-life assessment and functional status) |
| Health economic analysis comparing cost-effectiveness |
| Outcome measures were defined in accordance with the updated Valve Academic Research Consortium-2 consensus document10. Primary and major secondary endpoints are listed. |
Statistical analysis
All primary analyses will be performed in the intention-to-treat population, which includes all randomised patients by their allocated treatment. The multiple testing strategy for the 2 co-primary and the first 3 secondary endpoints is laid out in Figure 2 using the graphical concept of hierarchical procedures11. In the first step, non-inferiority by the same ratio is tested for both safety at 1 year after randomisation and efficacy at 5 years after randomisation. To this end, Cox models stratified by STS-PROM score are used to estimate the cause-specific hazard ratios (HR) restricted to the respective follow-up. Patients lost to follow-up and patients with administrative censoring are treated identically, with the assumption of non-informative censoring. If non-inferiority is shown for both safety after 1 year and efficacy after 5 years, each at the 1-sided 2.5% test level using the log-rank test, superiority at 5 years after randomisation will be tested at a 2-sided level of 5% using the Cox model stratified by STS-PROM score.
To quantify survival benefits, differences in the restricted mean survival times (RMST) will be estimated. Specifically, we will test whether the RMST differs over the period from randomisation until 5-year follow-up, from randomisation until 1-year follow-up, and from 1 year to 5 years after randomisation. The RMST tests are embedded in the hierarchical testing procedure described in Figure 2.
Sensitivity analyses will be performed with stratification by periods of constant eligibility and lockdown for the coronavirus disease (COVID-19) pandemic. For all endpoints, 95% confidence intervals are not adjusted for multiple comparisons. Competing risk models are used to estimate cumulative incidence curves for the secondary endpoints. Predefined subgroup analyses will include age, sex, New York Heart Association (NYHA) Class, transcatheter heart valve (THV)/prosthesis type, access route, relevant baseline comorbidities, STS-PROM strata, accrual periods of constant eligibility, and lockdown for the COVID-19 pandemic, among other data (Supplementary Appendix 2). The latter two were included in the statistical analysis plan after the start of the COVID-19 pandemic. Safety analyses are performed parallel with treatment.
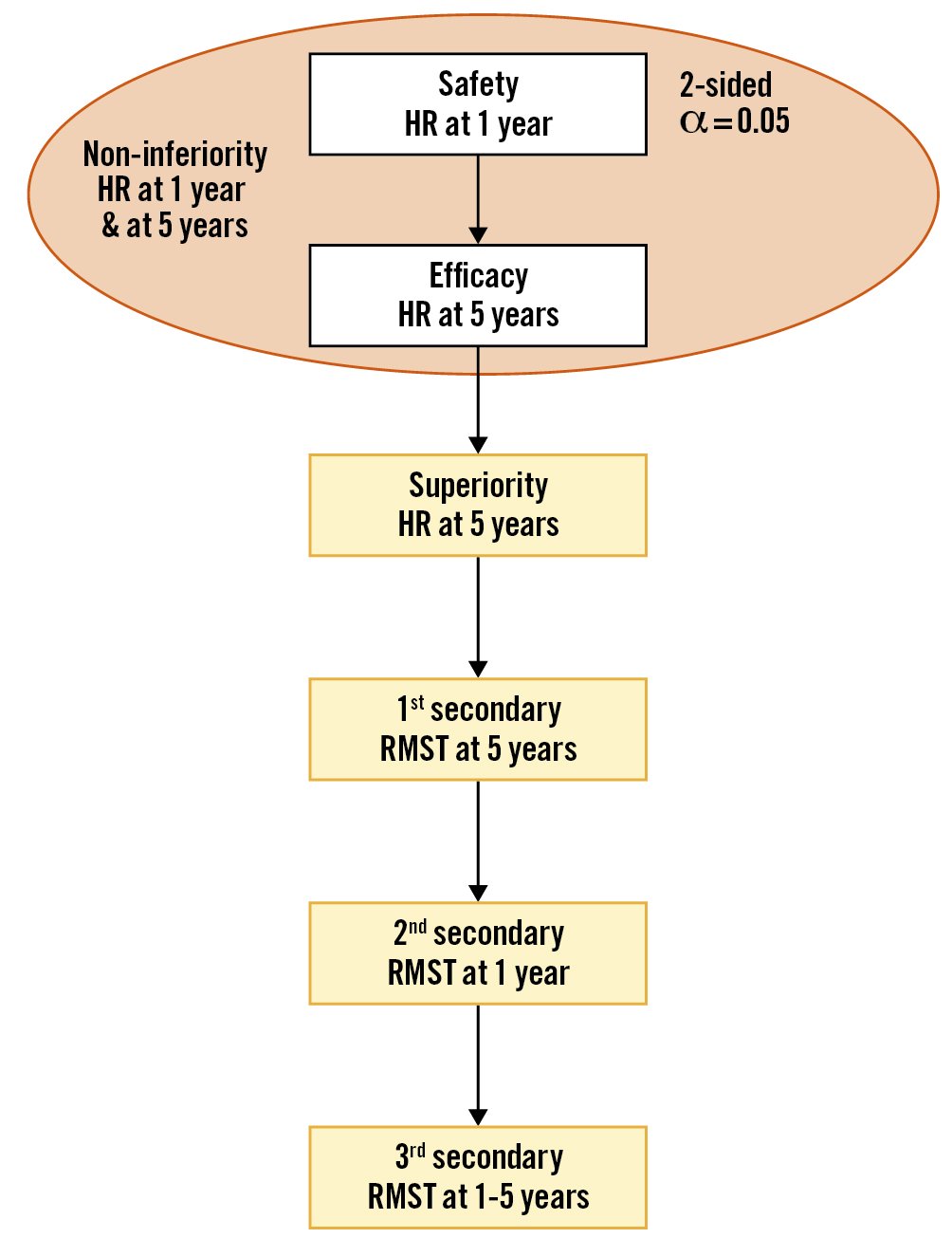
Figure 2. Statistical testing strategy. In the first step, non-inferiority is tested for both safety at 1 year and efficacy at 5 years after randomisation using the hazard ratio (HR). Both hypotheses need to show non-inferiority at the 2-sided 0.05 test level, i.e., 0.025 1-sided, for continuation of the test procedure. If both tests show non-inferiority, the full significance level of 0.05 is transferred for superiority testing at 5 years after randomisation. All tests for the restricted mean survival time (RMST) are superiority tests at the 2-sided 0.05 test level and are only conducted when all previous tests in this hierarchical testing strategy show significance.
Planned sample size
At the time that the trial was designed, data were available from only 3 RCTs which included primarily intermediate risk strata or a smaller sample size121314. The expected event rates were based on these data; they were subsequently modified to include general age-related mortalities and STS-PROM scores when patients with lower operative risk were included. The initial 1-year mortality was expected to be 7.8% among patients after TAVI and 11.4% among patients after SAVR. More recent RCTs have suggested far lower event rates and hazard ratios (HR) than we had initially used for our sample size calculation45615. Based on these contemporary data and a blinded interim analysis of the DEDICATE-DZHK6 Trial after recruitment of 881 patients, we assumed the geometric mean 1-year rate of mortality or stroke to be 6.2%. The non-inferiority margin was adjusted from HR 1.10 to HR 1.14 so that the rejectable difference of proportions at 1 year remained 1 percentage point. The enrolment of approximately 1,404 patients provides a power of 80% to reject the non-inferiority margin at 1 year for the alternative HR of 0.67 when the censoring rate was 10% per year. The same assumptions and rates of recruitment and of events, stratified by risk classes estimated at blinded interim analysis, gave a power of 94% at 5 years, which translates to a power of 76% for rejecting equal hazards in the superiority test of efficacy.
Discussion
Building on current evidence for TAVI and SAVR in patients with symptomatic severe aortic stenosis, DEDICATE-DZHK6 should provide additional data to help further define the optimal treatment strategies. Particularly for younger, low-risk patients who are amenable to both therapies, the evidence needed to inform treatment decisions with respect to longer-term outcomes is not fully established. DEDICATE-DZHK6 evaluates the impact of the treatment strategy on the primary endpoints of all-cause mortality and stroke at 1 year (co-primary safety endpoint) and 5 years (primary efficacy endpoint). The 5-year time frame for the primary endpoint ensures that early midterm results will weigh into the primary outcome of the trial and complement early 1-year safety data. A particular strength of the trial is its strict statistical analysis. The set non-inferiority margin corresponds to an absolute difference of event rates of approximately 1% at 1 year, while it was set as wide as 5-6% in most other trials456. A relevant risk difference of 2% would correspond to one-third of the average event rate at 1 year, which is a common value to detect clinically relevant differences and corresponds well with the alternative hypothesis of this trial. Overinterpretation of insignificant results is prevented by calculating confidence limits for several estimates. As some previous trials have indicated crossing hazards during follow-up, with lower early event rates after TAVI compared to SAVR, followed by higher event rates during the non-prespecified observation period1617, we decided to cover this aspect by using prespecified time frames for the primary endpoint.
Currently, robust data on the long-term durability of THVs remain scarce. The majority of systematic 5-year follow-up data stem from RCTs that enrolled older, intermediate- and high-risk patient populations1618192021; few data are available up to 8 years7. Although current data demonstrate the durability of TAVI and SAVR to be comparable in the respective time frames, their applicability to younger, low-risk patients remains unclear, as the competing risk of mortality may mask structural valve deterioration. Furthermore, variable definitions of structural valve deterioration complicate the systematic evaluation of this important aspect. A systematic 10-year follow-up is planned for the most recent low-risk trials1722; this will add important information on durability and subsequent decision-making in younger patients with a long life expectancy.
DEDICATE-DZHK6 aims to investigate treatment of isolated aortic valve disease in an all-comers patient population. The trial was designed with broad eligibility criteria, putting the local interdisciplinary Heart Team at the core of the enrolment process. If the local Heart Team agreed on the patient´s eligibility for both treatment strategies, isolated SAVR and TAVI, inclusion into the trial was recommended. The majority of RCTs in this field were planned to evaluate the performance of TAVI with one specific THV prosthesis compared to SAVR, while DEDICATE-DZHK6 was designed to compare the two treatment strategies. Periprocedural aspects, the choice of the valve prosthesis or access, antithrombotic management, and further treatment-related medical decisions were left to the discretion of the local Heart Team in order to tailor the assigned strategy to the individual patients’ anatomies and comorbidities.
DEDICATE-DZHK6 is an industry-independent study, conceptualised to mirror clinical reality and provide unique information on overall outcomes that can be directly applied to clinical routine. Hence, together with the other ongoing RCTs in this field, DEDICATE-DZHK6 may help to shape treatment strategies for low-risk patients with severe symptomatic aortic stenosis in the near future.
Limitations
At the time of the trial design, there was a paucity of outcome data in low- to intermediate-risk patients to estimate event rates for DEDICATE-DZHK6. As new evidence for TAVI in these patient populations became available during the enrolment period45615 and guidelines for the treatment of valvular heart disease were updated1, the study protocol was amended to accommodate evolving clinical practice patterns and ensure patient recruitment while retaining sufficient statistical power. While the trial had initially been conceptualised to primarily include patients at intermediate operative risk, we subsequently amended the protocol to enrol all-comer patients at low to intermediate risk. Overall, DEDICATE-DZHK6 represents a routine low- to intermediate-risk patient population. A blinded interim analysis was performed to confirm sufficient power and sample size calculations, and any changes made will be incorporated within the statistical analyses. The COVID-19 pandemic may have altered treatment strategies of elective cases over a relevant period of the recruitment period and may generally have impacted patient outcomes. Secondary analyses will be performed to address these unforeseen challenges. DEDICATE-DZHK6 targeted an all-comers tricuspid aortic stenosis population. Patients with bicuspid aortic stenoses or concomitant clinically relevant coronary or other valvular heart disease were not enrolled. As the majority of patients had already been enrolled at the time of publication of the updated VARC-3 criteria23, we proceeded with clinical event adjudication according to the VARC-2 document10.
Conclusions
The DEDICATE-DZHK6 Trial is an investigator-initiated, industry-independent and pragmatic German multicentre, randomised controlled study comparing TAVI and SAVR in low- to intermediate-risk patients targeting mortality or stroke at 1 and 5 years as the primary safety and efficacy outcomes. It will build on current scientific and medical evidence. Its results will support medical decisions to further define optimal treatment strategies for patients with severe aortic stenosis in whom both TAVI and SAVR are advisable.
Acknowledgements
The trial was carried out using the clinical research platform of the DZHK (German Centre for Cardiovascular Research).
Funding
DEDICATE-DZHK6 is an investigator-initiated trial. Funding is provided exclusively by the DZHK (German Centre for Cardiovascular Research, funding code: 81X1710106) and the German Heart Foundation (Deutsche Herzstiftung e.V., Frankfurt am Main), assuring the independence of the trial.
Conflict of interest statement
M. Seiffert received speaker or advisory fees from Abbott Vascular, Abiomed, Amgen, AstraZeneca, Boston Scientific, Bristol-Myers Squibb, Daichii Sankyo, Edwards Lifesciences, Inari Medical, Medtronic, Pfizer, Shockwave Medical, and Siemens Healthineers; and aresearch grant from Boston Scientific - all unrelated to the submitted work. M. Borger declares that his hospital receives speaker honoraria and/or consulting fees on his behalf from Edwards Lifesciences, Medtronic, Abbott, and Artivion. V. Falk has relevant financial activities outside the submitted work with following commercial entities: Medtronic, Biotronik, Abbott, Boston Scientific, Edwards Lifesciences, LivaNova, Berlin Heart, Novartis, JOTEC/Artivion, and Zurich Heart. C. Hamm is amember of the International Strategic Advisory Board at Medtronic. U. Landmesser reports grants to institution from Bayer, Amgen, and Novartis; and speaker or advisory fees from Abbott and Boston Scientific. H. Reichenspurner is amember of the advisory board at Medtronic; and declares that he receives speaker honoraria from Abiomed and Abbott. R. Twerenbold holds aprofessorship in clinical cardiology at the University Medical Center Hamburg-Eppendorf, supported by the Kühne Foundation; reports research support from the German Centre for Cardiovascular Research (DZHK) and the Swiss National Science Foundation (Grant No. P300PB_167803), speaker/consulting honoraria from Abbott, Amgen, AstraZeneca, Psyros, Roche, Siemens, Singulex, and Thermo Scientific BRAHMS, outside the submitted work. S. Blankenberg, R. Twerenbold and A. Ziegler are listed as co-inventors of an international patent on the use of acomputing device to estimate the probability of myocardial infarction (International Publication Number WO2022043229A1) as well as co-founders and shareholders of ART-EMIS Hamburg GmbH. A. Ziegler is ascientific director of Cardio-CARE, which is another shareholder of ART-EMIS Hamburg GmbH. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

